An intranasal vaccine targeting the receptor binding domain of SARS-CoV-2 elicits a protective immune response
- PMID: 36466882
- PMCID: PMC9708728
- DOI: 10.3389/fimmu.2022.1005321
An intranasal vaccine targeting the receptor binding domain of SARS-CoV-2 elicits a protective immune response
Abstract
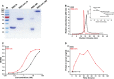
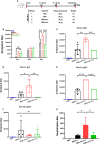
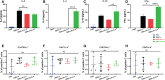
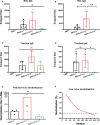
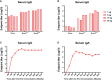
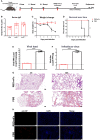
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the pathogen responsible for COVID-19, has caused an ongoing worldwide pandemic. Due to the rapid emergence of variants of concern (VOCs), novel vaccines and vaccination strategies are urgently needed. We developed an intranasal vaccine consisting of the SARS-CoV-2 receptor binding domain (RBD) fused to the antibody Fc fragment (RBD-Fc). RBD-Fc could induce strong humoral immune responses via intranasal vaccination. Notably, this immunogen could efficiently induce IgG and IgA and establish mucosal immunity in the respiratory tract. The induced antibodies could efficiently neutralize wild-type SARS-CoV-2 and currently identified SARS-CoV-2 VOCs, including the Omicron variant. In a mouse model, intranasal immunization could provide complete protection against a lethal SARS-CoV-2 challenge. Unfortunately, the limitation of our study is the small number of animals used in the immune response analysis. Our results suggest that recombinant RBD-Fc delivered via intranasal vaccination has considerable potential as a mucosal vaccine that may reduce the risk of SARS-CoV-2 infection.
Keywords: RBD-Fc; SARS-CoV-2; intranasal vaccine; mucosal immunity; mucosal vaccine.
Copyright © 2022 Chen, Zhang, Li, Wu, Zhang and Gong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

