Differential diagnosis and identification of prognostic markers for peripheral T-cell lymphoma subtypes based on flow cytometry immunophenotype profiles
- PMID: 36466894
- PMCID: PMC9715969
- DOI: 10.3389/fimmu.2022.1008695
Differential diagnosis and identification of prognostic markers for peripheral T-cell lymphoma subtypes based on flow cytometry immunophenotype profiles
Abstract
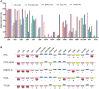
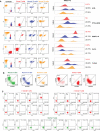
We compared the differential expression of 15 markers in PTCL (Peripheral T-cell lymphoma) subtypes and T-CUS (T-cell clones of uncertain significance), and summarized the specific immunophenotype profiles of each subtype and its impact on prognosis. PD-1 and CD10 are diagnostic markers for AITL (angioimmunoblastic T-cell lymphoma). To avoid confusion with T-CUS of benign clones, it is recommended to define AITL as bounded by PD-1+%>38.01 and/or CD10+%>7.46. T cell-derived ENKTL-N (extranodal NKT cell lymphoma) specifically expresses CD56. ALCL (anaplastic large cell lymphoma) characteristically expresses CD30 and HLA-DR. PTCL-NOS (peripheral T-cell lymphoma unspecified) still lacks a relatively specific phenotype and is prone to loss of basic lineage markers CD3, CD5, and CD7. The determination of T-CUS can be verified by the overall assessment of the bone marrow and a certain period of follow-up. The clustering results showed that the expression of 8 specific markers was significantly different among the 5 groups, suggesting that a combination of related markers can be analyzed in the identification of PTCLs subtypes. The study explores the advantages of TRBC1 combined with CD45RA/CD45RO in detecting T cell clonality, which can efficiently and sensitively analyze multiple target T cell populations at the same time. The sensitivity of PB to replace BM to monitor the tumor burden or MRD (minimal residual disease) of PTCLs is as high as 85.71%, which can relieve the huge pressure of clinical sampling and improve patient compliance. CD7, CD38, and Ki-67 are prognostic indicators for AITL. CD3 and CD8 on PTCL-NOS, and CD56 and HLA-DR on ENKTL-N have prognostic role. This study supports and validates the current classification of PTCL subtypes and establishes an immunophenotypic profile that can be used for precise diagnosis. The important clinical value of PTCLs immunophenotype in routine classification diagnosis, clonality confirmation, prognosis prediction, and treatment target selection was emphasized.
Keywords: AITCL; FCM immunophenotype; T-CUS; differential diagnosis; peripheral T-cell lymphoma; prognostic marker; targeted therapy.
Copyright © 2022 Pu, Qiao, Liu, Cao, Tan, Yan, Wang, Li and Yue.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Mourad N, Mounier N, Brière J, Raffoux E, Delmer A, Feller A, et al. . Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the groupe d'etude des lymphomes de l'adulte (Gela) trials. Blood (2008) 111(9):4463–70. doi: 10.1182/blood-2007-08-105759 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

