Low fish meal diet supplemented with probiotics ameliorates intestinal barrier and immunological function of Macrobrachium rosenbergii via the targeted modulation of gut microbes and derived secondary metabolites
- PMID: 36466900
- PMCID: PMC9713824
- DOI: 10.3389/fimmu.2022.1074399
Low fish meal diet supplemented with probiotics ameliorates intestinal barrier and immunological function of Macrobrachium rosenbergii via the targeted modulation of gut microbes and derived secondary metabolites
Abstract
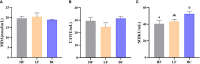
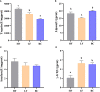
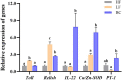
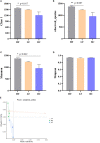
The unsuitable substitution ratio of fish meal by plant protein will reshape the intestinal microbial composition and intestine immunity. However, previous studies were mostly limited to investigating how different feed or probiotics characterized the microbial composition but ignored the biological interactions between bacteria and host physiology through secondary metabolites. Therefore, this study integrates the apparent indicators monitoring, 16S rDNA sequencing, and metabonomics to systematically investigate the effects of cottonseed protein concentrate (CPC) substitution of fish meal and Bacillus coagulans intervention on gut microbes, secondary metabolites, and intestinal immunity of Macrobrachium rosenbergii. Prawns were fed with three diets for 70 days: HF diets contained 25% fish meal, CPC in LF diets were replaced with 10% fish meal, and LF diets supplemented with 2 × 108 CFU/g diet B. coagulans were designated as BC diets. Results showed that CPC substitution induced a significant decrease in digestive enzyme activities (trypsin and lipase) and gut barrier protein PT-1 expression and a significant increase in γ-GT enzyme activity and inflammatory-related factors (Relish and Toll) expression. B. coagulans treatment mitigated the negative changes of the above indicators. Meanwhile, it significantly improved the expression levels of the barrier factor PT-1, the reparative cytokine IL-22, and Cu/Zn-SOD. CPC substitution resulted in a remarkable downregulated abundance of Firmicutes phyla, Flavobacterium spp., and Bacillus spp. B. coagulans treatment induced the callback of Firmicutes abundance and improved the relative abundance of Sphingomonas, Bacillus, and Ralstonia. Functional prediction indicated that CPC substitution resulted in elevated potential pathogenicity of microbial flora, and B. coagulans reduces the pathogenesis risk. Pearson's correlation analysis established a significant positive correlation between differential genera (Sphingomonas, Bacillus, and Ralstonia) and secondary metabolites (including sphingosine, dehydrophytosphingosine, amino acid metabolites, etc.). Meanwhile, the latter were significantly associated with intestinal immunoregulation-related genes (Cu/Zn-SOD, IL-22, PT-1, Toll, and Relish). This study indicated that B. coagulans could mediate specific gut microbes and the combined action of multiple functional secondary metabolites to affect intestinal barrier function, digestion, and inflammation. Our study revealed the decisive role of gut microbes and derived secondary metabolites in the model of dietary composition-induced intestinal injury and probiotic treatment from a new perspective.
Keywords: Macrobrachium rosenbergii; bacillus coagulans; cottonseed protein concentrate; fish meal; intestinal microbial; secondary metabolites.
Copyright © 2022 Zheng, Liu, Wang, Yang, Zhou, Sun and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Supplementation of Yupingfeng polysaccharides in low fishmeal diets enhances intestinal health through influencing the intestinal barrier, immunity, and microflora in Macrobrachium rosenbergii.Front Immunol. 2024 Nov 26;15:1480897. doi: 10.3389/fimmu.2024.1480897. eCollection 2024. Front Immunol. 2024. PMID: 39660141 Free PMC article.
-
Bile acid and short chain fatty acid metabolism of gut microbiota mediate high-fat diet induced intestinal barrier damage in Macrobrachium rosenbergii.Fish Shellfish Immunol. 2024 Mar;146:109376. doi: 10.1016/j.fsi.2024.109376. Epub 2024 Jan 11. Fish Shellfish Immunol. 2024. PMID: 38218421
-
Cottonseed protein concentrate as an effective substitute to fish meal in pike perch (Sander luciperca) feed: evidence from growth performance and intestinal responses of immune function and microflora.Front Immunol. 2025 Mar 4;16:1522005. doi: 10.3389/fimmu.2025.1522005. eCollection 2025. Front Immunol. 2025. PMID: 40109349 Free PMC article.
-
Progress of research and application of Heyndrickxia coagulans (Bacillus coagulans) as probiotic bacteria.Front Cell Infect Microbiol. 2024 May 28;14:1415790. doi: 10.3389/fcimb.2024.1415790. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38863834 Free PMC article. Review.
-
Effects of Probiotics on Gut Microbiota: An Overview.Int J Mol Sci. 2024 May 30;25(11):6022. doi: 10.3390/ijms25116022. Int J Mol Sci. 2024. PMID: 38892208 Free PMC article. Review.
Cited by
-
Lactobacillus rhamnosus GG ameliorates osteoporosis in ovariectomized rats by regulating the Th17/Treg balance and gut microbiota structure.Gut Microbes. 2023 Jan-Dec;15(1):2190304. doi: 10.1080/19490976.2023.2190304. Gut Microbes. 2023. PMID: 36941563 Free PMC article.
-
Understanding the role of microbes in health and disease of farmed aquatic organisms.Mar Life Sci Technol. 2024 Sep 19;6(4):579-609. doi: 10.1007/s42995-024-00248-8. eCollection 2024 Nov. Mar Life Sci Technol. 2024. PMID: 39620093
-
Dietary Administration of Postbiotics from Vibrio proteolyticus DCF12.2 Enhanced Intestinal Integrity, Microbiota, and Immune Response in Juvenile Gilthead Seabream (Sparus aurata).Animals (Basel). 2025 Jul 5;15(13):1982. doi: 10.3390/ani15131982. Animals (Basel). 2025. PMID: 40646881 Free PMC article.
-
Probiotic Bacillus licheniformis ZW3 Alleviates DSS-Induced Colitis and Enhances Gut Homeostasis.Int J Mol Sci. 2024 Jan 1;25(1):561. doi: 10.3390/ijms25010561. Int J Mol Sci. 2024. PMID: 38203732 Free PMC article.
-
The Bile Acid Metabolism of Intestinal Microorganisms Mediates the Effect of Different Protein Sources on Muscle Protein Deposition in Procambarus clarkii.Microorganisms. 2024 Dec 24;13(1):11. doi: 10.3390/microorganisms13010011. Microorganisms. 2024. PMID: 39858779 Free PMC article.
References
-
- Bian F, Zhou H, He G, Wang C, Peng H, Pu X, et al. . Effects of replacing fishmeal with different cottonseed meals on growth, feed utilization, haematological indexes, intestinal and liver morphology of juvenile turbot (Scophthalmus maximus l.) . Aquacult Nutr (2017) 23(6):1429–39. doi: 10.1111/anu.12518 - DOI
-
- Olsen RL, Hasan MR. A limited supply of fishmeal: Impact on future increases in global aquaculture production. Trends Food Sci Technol (2012) 27(2):120–8. doi: 10.1016/j.tifs.2012.06.003 - DOI
-
- Cavrois-Rogacki T, Leeming D, Lopez PM, Davie A, Migaud H. Plant-based protein ingredients can successfully replace fish meal in the diet of ballan wrasse (LABRUS BERGYLTA) juveniles. Aquaculture (2022) 546:737419. doi: 10.1016/j.aquaculture.2021.737419 - DOI
-
- Zhang W, Tan BP, Ye GL, Wang JX, Dong XH, Yang QH, et al. . Identification of potential biomarkers for soybean meal-induced enteritis in juvenile pearl gentian grouper, epinephelus lanceolatus♂ × epinephelus fuscoguttatus♀. Aquaculture (2019) 512:734337. doi: 10.1016/j.aquaculture.2019.734337 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

