The role of cholesterol and mitochondrial bioenergetics in activation of the inflammasome in IBD
- PMID: 36466902
- PMCID: PMC9716353
- DOI: 10.3389/fimmu.2022.1028953
The role of cholesterol and mitochondrial bioenergetics in activation of the inflammasome in IBD
Erratum in
-
Corrigendum: The role of cholesterol and mitochondrial bioenergetics in activation of the inflammasome in IBD.Front Immunol. 2024 Feb 26;15:1384162. doi: 10.3389/fimmu.2024.1384162. eCollection 2024. Front Immunol. 2024. PMID: 38469317 Free PMC article.
Abstract
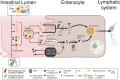
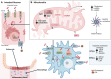
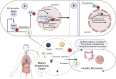
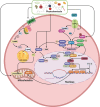
Inflammatory Bowel Disease (IBD) is characterized by a loss of intestinal barrier function caused by an aberrant interaction between the immune response and the gut microbiota. In IBD, imbalance in cholesterol homeostasis and mitochondrial bioenergetics have been identified as essential events for activating the inflammasome-mediated response. Mitochondrial alterations, such as reduced respiratory complex activities and reduced production of tricarboxylic acid (TCA) cycle intermediates (e.g., citric acid, fumarate, isocitric acid, malate, pyruvate, and succinate) have been described in in vitro and clinical studies. Under inflammatory conditions, mitochondrial architecture in intestinal epithelial cells is dysmorphic, with cristae destruction and high dynamin-related protein 1 (DRP1)-dependent fission. Likewise, these alterations in mitochondrial morphology and bioenergetics promote metabolic shifts towards glycolysis and down-regulation of antioxidant Nuclear erythroid 2-related factor 2 (Nrf2)/Peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α) signaling. Although the mechanisms underlying the mitochondrial dysfunction during mucosal inflammation are not fully understood at present, metabolic intermediates and cholesterol may act as signals activating the NLRP3 inflammasome in IBD. Notably, dietary phytochemicals exhibit protective effects against cholesterol imbalance and mitochondrial function alterations to maintain gastrointestinal mucosal renewal in vitro and in vivo conditions. Here, we discuss the role of cholesterol and mitochondrial metabolism in IBD, highlighting the therapeutic potential of dietary phytochemicals, restoring intestinal metabolism and function.
Keywords: IBD - inflammatory bowel disease; NLRP3 inflammasome; diet phytochemicals; inflammasome; intracellular cholesterol accumulation; mitochondrial dysfunction.
Copyright © 2022 Astorga, Gasaly, Dubois-Camacho, De la Fuente, Landskron, Faber, Urra and Hermoso.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Malvidin promotes PGC-1α/Nrf2 signaling to attenuate the inflammatory response and restore mitochondrial activity in septic acute kidney injury.Chem Biol Interact. 2024 Jan 25;388:110850. doi: 10.1016/j.cbi.2023.110850. Epub 2023 Dec 20. Chem Biol Interact. 2024. PMID: 38135199
-
Mitochondrial biogenesis: pharmacological approaches.Curr Pharm Des. 2014;20(35):5507-9. doi: 10.2174/138161282035140911142118. Curr Pharm Des. 2014. PMID: 24606795
-
Mitochondrial dysfunction induced by HIF-1α under hypoxia contributes to the development of gastric mucosal lesions.Clin Transl Med. 2024 Apr;14(4):e1653. doi: 10.1002/ctm2.1653. Clin Transl Med. 2024. PMID: 38616702 Free PMC article.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
-
Gut bacteria signaling to mitochondria in intestinal inflammation and cancer.Gut Microbes. 2020 May 3;11(3):285-304. doi: 10.1080/19490976.2019.1592421. Epub 2019 Mar 26. Gut Microbes. 2020. PMID: 30913966 Free PMC article. Review.
Cited by
-
TGR5 deficiency in excitatory neurons ameliorates Alzheimer's pathology by regulating APP processing.Sci Adv. 2024 Jun 28;10(26):eado1855. doi: 10.1126/sciadv.ado1855. Epub 2024 Jun 28. Sci Adv. 2024. PMID: 38941459 Free PMC article.
-
Maternal Intake of Laminarin Improves Infant Growth and Health by Fortifying Metabolite Profiles of Colostrum and Milk.J Agric Food Chem. 2024 Nov 27;72(47):26178-26188. doi: 10.1021/acs.jafc.4c07560. Epub 2024 Nov 14. J Agric Food Chem. 2024. PMID: 39542438
-
Intestinal microflora promotes Th2-mediated immunity through NLRP3 in damp and heat environments.Front Immunol. 2024 May 2;15:1367053. doi: 10.3389/fimmu.2024.1367053. eCollection 2024. Front Immunol. 2024. PMID: 38756775 Free PMC article.
-
Drp1: Focus on Diseases Triggered by the Mitochondrial Pathway.Cell Biochem Biophys. 2024 Jun;82(2):435-455. doi: 10.1007/s12013-024-01245-5. Epub 2024 Mar 4. Cell Biochem Biophys. 2024. PMID: 38438751 Review.
-
DAMP-ing IBD: Extinguish the Fire and Prevent Smoldering.Dig Dis Sci. 2025 Jan;70(1):49-73. doi: 10.1007/s10620-024-08523-5. Epub 2024 Jul 4. Dig Dis Sci. 2025. PMID: 38963463 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

