Foxp3+ regulatory T cell therapy for tolerance in autoimmunity and solid organ transplantation
- PMID: 36466912
- PMCID: PMC9714335
- DOI: 10.3389/fimmu.2022.1055466
Foxp3+ regulatory T cell therapy for tolerance in autoimmunity and solid organ transplantation
Abstract
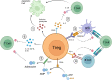
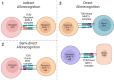
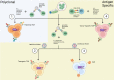
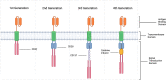
Regulatory T cells (Tregs) are critical for tolerance in humans. The exact mechanisms by which the loss of peripheral tolerance leads to the development of autoimmunity and the specific role Tregs play in allograft tolerance are not fully understood; however, this population of T cells presents a unique opportunity in the development of targeted therapeutics. In this review, we discuss the potential roles of Foxp3+ Tregs in the development of tolerance in transplantation and autoimmunity, and the available data regarding their use as a treatment modality.
Keywords: adoptive cell transfer; allograft tolerance; antigen-specific regulatory T cells; autoimmunity; regulatory T cells; solid organ transplantation.
Copyright © 2022 Sanders, Jeyamogan, Mathew and Leventhal.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

