Real-World Experience with Nivolumab in Metastatic Renal Cell Carcinoma Patients Who Have Progressed on Prior Therapies: A Single-Center Study from India
- PMID: 36466979
- PMCID: PMC9718606
- DOI: 10.1055/s-0041-1740373
Real-World Experience with Nivolumab in Metastatic Renal Cell Carcinoma Patients Who Have Progressed on Prior Therapies: A Single-Center Study from India
Abstract
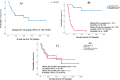
Amit RauthanIntroduction Nivolumab monotherapy is approved for the treatment of metastatic renal cell carcinoma (mRCC) patients who have progressed on prior therapies based on the pivotal Checkmate-025 trial. There is limited literature on the efficacy and safety profile of usage of nivolumab in the treatment of mRCC in India in a real-world setting. Methods A retrospective analysis was performed of patients who received nivolumab monotherapy for mRCC after having progressed on prior therapies. Tumor response was graded according to RECIST v1.1 and Kaplan-Meier survival analysis was used to estimate progression-free survival (PFS) and overall survival (OS). Immune-related adverse events (irAEs) were documented and graded according to CTCAE v5.0. Results Between 2016 and 2019, 35 patients received nivolumab for mRCC at our center after progression on prior therapies. A majority of the patients ( n = 30, 85.7%) received it in a second-line setting, and the remaining in the third line and beyond setting. Clear cell was the most common histology ( n = 26, 74.3%). There were 18 patients (51.42%) who belonged to IMDC intermediate risk, while 17 (48.58%) patients were at poor risk. The overall response rate was 60%, with complete response (CR) in 11.4%. Median duration of response was not reached among responders. Median PFS was 5 months (95% confidence interval [CI]: 3.06-6.93) and median OS was 26 months (95% CI: 1.90-50.09). Ongoing survival of 47, 42, 34, and 22 months was noted in four patients with CR, respectively. In our study, 23 patients (65.71%) experienced any grade of irAE. Grade 3 irAEs was seen in four patients (11.42%). Most common irAE was thyroid dysfunction seen in 12 patients (34.2%). Treatment discontinuation due to irAEs occurred in three patients (8.57%). Conclusion Nivolumab showed good efficacy with high response rates and an OS comparable to the pivotal Checkmate-025 trial. It was well tolerated with safety profile in terms of irAE consistent with those reported in literature.
Keywords: Indian experience; immunotherapy; irAEs; metastatic RCC; nivolumab.
MedIntel Services Pvt Ltd. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. ( https://creativecommons.org/licenses/by-nc-nd/4.0/ ).
Conflict of interest statement
Conflict of Interest None declared.
Figures
References
-
- Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F.Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer [Internet] 2020[cited 2021 Apr 10]. Accessed September 30, 2021 from:https://gco.iarc.fr/today
-
- Motzer R J, Hutson T E, Tomczak P. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(02):115–124. - PubMed
-
- Sternberg C N, Davis I D, Mardiak J. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28(06):1061–1068. - PubMed
-
- TARGET Study Group . Escudier B, Eisen T, Stadler W M. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(02):125–134. - PubMed
LinkOut - more resources
Full Text Sources