Treatment of ADHD: Drugs, psychological therapies, devices, complementary and alternative methods as well as the trends in clinical trials
- PMID: 36467081
- PMCID: PMC9713849
- DOI: 10.3389/fphar.2022.1066988
Treatment of ADHD: Drugs, psychological therapies, devices, complementary and alternative methods as well as the trends in clinical trials
Abstract
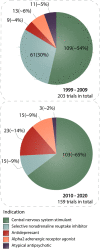
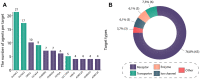
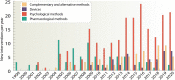
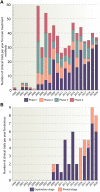
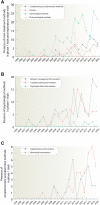
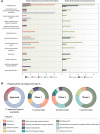
Attention-deficit/hyperactivity disorder (ADHD) is one of the most common neurodevelopmental disorders having a high influence on social interactions. The number of approved treatments and clinical trials for ADHD have increased markedly during the recent decade. This analytical review provides a quantitative overview of the existing pharmacological and non-pharmacological methods of ADHD treatments investigated in clinical trials during 1999-2021. A total of 695 interventional trials were manually assessed from clinicaltrial.gov with the search term « ADHD», and trial data has been used for analysis. A clear majority of the studies investigated non-pharmacological therapies (∼80%), including many behavioral options, such as social skills training, sleep and physical activity interventions, meditation and hypnotherapy. Devices, complementary and other alternative methods of ADHD treatment are also gaining attention. The pharmacological group accounts for ∼20% of all the studies. The most common drug classes include central nervous system stimulants (e.g., methylphenidate hydrochloride, lisdexamfetamine dimesylate, amphetamine sulfate, mixed amphetamine salts, a combination of dexmethylphenidate hydrochloride and serdexmethylphenidate chloride), selective noradrenaline reuptake inhibitors (atomoxetine, viloxazine), and alpha2 adrenergic receptor agonists (guanfacine hydrochloride, clonidine hydrochloride). Several studies investigated antidepressants (e.g., bupropion hydrochloride, vortioxetine), and atypical antipsychotics (e.g., quetiapine, aripiprazole) but these are yet not approved by the FDA for ADHD treatment. We discuss the quantitative trends in clinical trials and provide an overview of the new drug agents and non-pharmacological therapies, drug targets, and novel treatment options.
Keywords: ADHD; agents; drugs; non-pharmacological; pharmacological; stimulants; treatment; trials.
Copyright © 2022 Nazarova, Sokolov, Chubarev, Tarasov and Schiöth.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Adler L. A., Adams J., Madera-McDonough J., Kohegyi E., Hobart M., Chang D., et al. (2022). Efficacy, safety, and tolerability of centanafadine sustained-release tablets in adults with attention-deficit/hyperactivity disorder: Results of 2 phase 3, randomized, double-blind, multicenter, placebo-controlled trials. J. Clin. Psychopharmacol. 42 (5), 429–439. 10.1097/JCP.0000000000001575 - DOI - PMC - PubMed
-
- APA (2013). Diagnostic and Statistical Manual of Mental Disorders. Arlington, VA, USA: American Psychiatric Publishing.
Publication types
LinkOut - more resources
Full Text Sources

