An orally available small molecule that targets soluble TNF to deliver anti-TNF biologic-like efficacy in rheumatoid arthritis
- PMID: 36467083
- PMCID: PMC9709720
- DOI: 10.3389/fphar.2022.1037983
An orally available small molecule that targets soluble TNF to deliver anti-TNF biologic-like efficacy in rheumatoid arthritis
Erratum in
-
Corrigendum: An orally available small molecule that targets soluble TNF to deliver anti-TNF biologic-like efficacy in rheumatoid arthritis.Front Pharmacol. 2024 Oct 16;15:1479557. doi: 10.3389/fphar.2024.1479557. eCollection 2024. Front Pharmacol. 2024. PMID: 39478966 Free PMC article.
Abstract
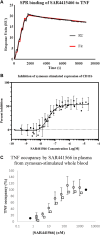
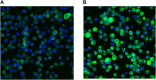
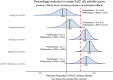
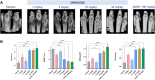
Tumor necrosis factor (TNF) is a pleiotropic cytokine belonging to a family of trimeric proteins with both proinflammatory and immunoregulatory functions. TNF is a key mediator in autoimmune diseases and during the last couple of decades several biologic drugs have delivered new therapeutic options for patients suffering from chronic autoimmune diseases such as rheumatoid arthritis and chronic inflammatory bowel disease. Attempts to design small molecule therapies directed to this cytokine have not led to approved products yet. Here we report the discovery and development of a potent small molecule inhibitor of TNF that was recently moved into phase 1 clinical trials. The molecule, SAR441566, stabilizes an asymmetrical form of the soluble TNF trimer, compromises downstream signaling and inhibits the functions of TNF in vitro and in vivo. With SAR441566 being studied in healthy volunteers we hope to deliver a more convenient orally bioavailable and effective treatment option for patients suffering with chronic autoimmune diseases compared to established biologic drugs targeting TNF.
Keywords: TNF; TNF trimer; immunogenicity; rheumatoid arthritis; small molecule.
Copyright © 2022 Vugler, O’Connell, Nguyen, Weitz, Leeuw, Hickford, Verbitsky, Ying, Rehberg, Carrington, Merriman, Moss, Nicholas, Stanley, Wright, Bourne, Foricher, Zhu, Brookings, Horsley, Heer, Schio, Herrmann, Rao, Kohlmann and Florian.
Conflict of interest statement
AV, JO’C, MN, DW, TL, EH, AVe, XY, MR, BC, MM, AM, J-MN, PS, SW, YF, ZZ, DB, HH, JH, LS, MH, SR, MK, and PF are either employees of UCB Pharma or Sanofi and may hold stock of the respective organizations. PF was employed by Sanofi-Aventis Deutschland GmbH at the time the work of the manuscript was conducted. He is now an employee of Boehringer Ingelheim Vetmedica GmbH. TM is employed by MilvuswoodConsultancy.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources

