Bushen huoxue decoction inhibits RANKL-stimulated osteoclastogenesis and glucocorticoid-induced bone loss by modulating the NF-κB, ERK, and JNK signaling pathways
- PMID: 36467086
- PMCID: PMC9716084
- DOI: 10.3389/fphar.2022.1007839
Bushen huoxue decoction inhibits RANKL-stimulated osteoclastogenesis and glucocorticoid-induced bone loss by modulating the NF-κB, ERK, and JNK signaling pathways
Erratum in
-
Corrigendum: Bushen huoxue decoction inhibits RANKL-stimulated osteoclastogenesis and glucocorticoid-induced bone loss by modulating the NF-κB, ERK, and JNK signaling pathways.Front Pharmacol. 2023 Feb 27;14:1148908. doi: 10.3389/fphar.2023.1148908. eCollection 2023. Front Pharmacol. 2023. PMID: 36925639 Free PMC article.
Abstract
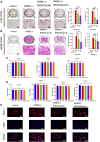
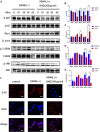
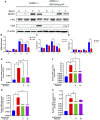
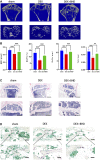
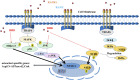
Glucocorticoid-induced osteoporosis (GIOP) is the most common form of secondary osteoporosis, which is caused by a disorder in bone metabolism due to excessive activation of osteoclasts. Bushen Huoxue decoction (BHD) is an herbal formula with multiple pharmacological effects, including anti-inflammatory, antioxidant activity and stem cell migration promotion. However, the effect of BHD on osteoclastogenesis has not been reported. In this study, we aimed to elucidate the effect of BHD on RANKL-stimulated osteoclastogenesis and explored its underlying mechanisms of action in vitro. Our results show that BHD had no effect on BMMs and RAW264.7 cells viability, but inhibited RANKL-induced osteoclast formation in vitro. Furthermore, BHD attenuated RANKL-induced NF-κB, ERK, and JNK signaling. The attenuation of NF-κB, ERK, and JNK activation were enough to impede downstream expression of c-fos and NFATc1 and related specific genes. Meanwhile, we investigated the therapeutic effect of BHD on glucocorticoid-induced osteoporosis (GIOP) mice. The result indicated that BHD prevents glucocorticoid-induced osteoporosis and preserves bone volume by repressing osteoclast activity. Collectively, BHD shows significant osteoclast inhibition and holds great promise in the treatment of osteoporosis.
Keywords: ERK pathway; JNK pathway; NF-κB pathway; bushen huoxue decoction; osteoporosis.
Copyright © 2022 Liu, Fu, Li, Chen, Li, Xu and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Corrigendum: Bushen huoxue decoction inhibits RANKL-stimulated osteoclastogenesis and glucocorticoid-induced bone loss by modulating the NF-κB, ERK, and JNK signaling pathways.Front Pharmacol. 2023 Feb 27;14:1148908. doi: 10.3389/fphar.2023.1148908. eCollection 2023. Front Pharmacol. 2023. PMID: 36925639 Free PMC article.
-
Leonurine hydrochloride inhibits osteoclastogenesis and prevents osteoporosis associated with estrogen deficiency by inhibiting the NF-κB and PI3K/Akt signaling pathways.Bone. 2015 Jun;75:128-37. doi: 10.1016/j.bone.2015.02.017. Epub 2015 Feb 21. Bone. 2015. PMID: 25708053
-
Tyloxapol inhibits RANKL-stimulated osteoclastogenesis and ovariectomized-induced bone loss by restraining NF-κB and MAPK activation.J Orthop Translat. 2021 Apr 10;28:148-158. doi: 10.1016/j.jot.2021.01.005. eCollection 2021 May. J Orthop Translat. 2021. PMID: 33981577 Free PMC article.
-
Caffeic acid 3,4-dihydroxy-phenethyl ester suppresses receptor activator of NF-κB ligand–induced osteoclastogenesis and prevents ovariectomy-induced bone loss through inhibition of mitogen-activated protein kinase/activator protein 1 and Ca2+–nuclear factor of activated T-cells cytoplasmic 1 signaling pathways.J Bone Miner Res. 2012 Jun;27(6):1298-1308. doi: 10.1002/jbmr.1576. J Bone Miner Res. 2012. PMID: 22337253
-
2E-Decene-4,6-diyn-1-ol-acetate inhibits osteoclastogenesis through mitogen-activated protein kinase-c-Fos-NFATc1 signalling pathways.Clin Exp Pharmacol Physiol. 2022 Mar;49(3):341-349. doi: 10.1111/1440-1681.13609. Epub 2021 Dec 30. Clin Exp Pharmacol Physiol. 2022. PMID: 34729812
Cited by
-
Mechanisms of action and synergetic formulas of plant-based natural compounds from traditional Chinese medicine for managing osteoporosis: a literature review.Front Med (Lausanne). 2023 Aug 28;10:1235081. doi: 10.3389/fmed.2023.1235081. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37700771 Free PMC article. Review.
-
Determination of the Combined Effects of Asian Herbal Medicine with Calcium and/or Vitamin D Supplements on Bone Mineral Density in Primary Osteoporosis: A Systematic Review and Meta-Analysis.Osteoporos Int. 2024 Jul;35(7):1-21. doi: 10.1007/s00198-024-07061-0. Epub 2024 Mar 13. Osteoporos Int. 2024. PMID: 38472336 Free PMC article.
-
Longbie capsules reduce bone loss in the subchondral bone of rats with comorbid osteoporosis and osteoarthritis by regulating metabolite alterations.Front Med (Lausanne). 2023 Oct 17;10:1256238. doi: 10.3389/fmed.2023.1256238. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37915330 Free PMC article.
References
-
- Abdelmagid S. M., Sondag G. R., Moussa F. M., Belcher J. Y., Yu B., Stinnett H., et al. (2015). Mutation in osteoactivin promotes receptor activator of NFκB ligand (RANKL)-mediated osteoclast differentiation and survival but inhibits osteoclast function. J. Biol. Chem. 290, 20128–20146. 10.1074/jbc.M114.624270 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

