Icotinib derivatives as tyrosine kinase inhibitors with anti-esophageal squamous carcinoma activity
- PMID: 36467103
- PMCID: PMC9709406
- DOI: 10.3389/fphar.2022.1028692
Icotinib derivatives as tyrosine kinase inhibitors with anti-esophageal squamous carcinoma activity
Abstract
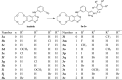
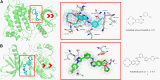
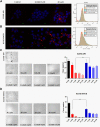
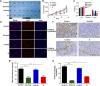
Previous report showed that a variety of icotinib derivatives bearing different 1,2,3-triazole moieties, which could be readily prepared via copper (I)-catalyzed cycloaddition (CuAAC) reaction between icotinib and different azides, exhibited interesting activity against different lung cancer cell lines such as H460, H1975, H1299, A549 or PC-9. To further expand the application scope of the compounds and to validate the function of triazole groups in drug design, the anti-cancer activity of these compounds against esophageal squamous carcinoma (ESCC) cells was tested herein. Preliminary MTT experiments suggested that these compounds were active against different ESCC cell lines such as KYSE70, KYSE410, or KYSE450 as well as their drug-resistant ones. Especially, compound 3l showed interesting anticancer activity against these cell lines. The mode of action was studied via molecular docking, SPR experiments and other biochemical studies, and 3l exhibited higher binding potential to wild-type EGFR than icotinib did. In vivo anticancer study showed that 3l could inhibit tumor growth of cell-line-derived xenografts in ESCC. Study also suggested that 3l was a potent inhibitor for EGFR-TK pathway. Combining these results, 3l represents a promising lead compound for the design of anti-cancer drugs against ESCC.
Keywords: 1,2,3-triazole; EGFR-TK pathway; ESCC; NSCLC; icotinib.
Copyright © 2022 Chen, Mao, Tian, Tian, Meng, Wang, Xu, Li, Liu and Dong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Anti-Resistant Strategies: Icotinib Derivatives as Promising Non-Small Cell Lung Cancer Therapeutics.Curr Cancer Drug Targets. 2025;25(5):483-495. doi: 10.2174/0115680096302595240605114828. Curr Cancer Drug Targets. 2025. PMID: 38967075
-
Design, synthesis and antitumor activity of icotinib derivatives.Bioorg Chem. 2020 Dec;105:104421. doi: 10.1016/j.bioorg.2020.104421. Epub 2020 Oct 22. Bioorg Chem. 2020. PMID: 33181408
-
Anti-tumor activity of high-dose EGFR tyrosine kinase inhibitor and sequential docetaxel in wild type EGFR non-small cell lung cancer cell nude mouse xenografts.Oncotarget. 2017 Feb 7;8(6):9134-9143. doi: 10.18632/oncotarget.13327. Oncotarget. 2017. PMID: 27852073 Free PMC article.
-
First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer.Cochrane Database Syst Rev. 2021 Mar 18;3(3):CD010383. doi: 10.1002/14651858.CD010383.pub3. Cochrane Database Syst Rev. 2021. PMID: 33734432 Free PMC article.
-
Clinical efficacy of icotinib in lung cancer patients with different EGFR mutation status: a meta-analysis.Oncotarget. 2017 May 16;8(20):33961-33971. doi: 10.18632/oncotarget.15475. Oncotarget. 2017. PMID: 28430623 Free PMC article. Review.
Cited by
-
Synthesis and In Vitro Antitumor Activity Evaluation of Gefitinib-1,2,3-Triazole Derivatives.Molecules. 2024 Feb 13;29(4):837. doi: 10.3390/molecules29040837. Molecules. 2024. PMID: 38398589 Free PMC article.
-
Anti-Resistant Strategies: Icotinib Derivatives as Promising Non-Small Cell Lung Cancer Therapeutics.Curr Cancer Drug Targets. 2025;25(5):483-495. doi: 10.2174/0115680096302595240605114828. Curr Cancer Drug Targets. 2025. PMID: 38967075
References
-
- Asokan S., Sridhar P., Qureshi M. M., Bhatt M., Truong M. T., Suzuki K., et al. (2020). Presentation, treatment, and outcomes of vulnerable populations with esophageal cancer treated at a safety-net hospital. Semin. Thorac. Cardiovasc. Surg. 32, 347–354. doi:. 10.1053/j.semtcvs.2019.12.008 - DOI - PubMed
-
- Chen X., Wu H., Chen H., Wang Q., Xie X. J., Shen J. (2019). Astragaloside VI promotes neural stem cell proliferation and enhances neurological function recovery in transient cerebral ischemic injury via activating EGFR/MAPK signaling cascades. Mol. Neurobiol. 56, 3053–3067. 10.1007/s12035-018-1294-3 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

