Safety and tolerability of bosutinib in patients with amyotrophic lateral sclerosis (iDReAM study): A multicentre, open-label, dose-escalation phase 1 trial
- PMID: 36467452
- PMCID: PMC9716331
- DOI: 10.1016/j.eclinm.2022.101707
Safety and tolerability of bosutinib in patients with amyotrophic lateral sclerosis (iDReAM study): A multicentre, open-label, dose-escalation phase 1 trial
Abstract
Background: Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease caused by the loss of motor neurons, and development of effective medicines is urgently required. Induced pluripotent stem cell (iPSC)-based drug repurposing identified the Src/c-Abl inhibitor bosutinib, which is approved for the treatment of chronic myelogenous leukemia (CML), as a candidate for the molecular targeted therapy of ALS.
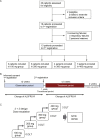
Methods: An open-label, multicentre, dose-escalation phase 1 study using a 3 + 3 design was conducted in 4 hospitals in Japan to evaluate the safety and tolerability of bosutinib in patients with ALS. Furthermore, the exploratory efficacy was evaluated using Revised ALS Functional Rating Scale (ALSFRS-R), predictive biomarkers including plasma neurofilament light chain (NFL) were explored, and single-cell RNA sequencing of iPSC-derived motor neurons was conducted. Patients, whose total ALSFRS-R scores decreased by 1-3 points during the 12-week, received escalating doses starting from 100 mg quaque die (QD) up to 400 mg QD based on dose-limiting toxicity (DLT) occurrence, and all participants who received one dose of the study drug were included in the primary analysis. This trial is registered with ClinicalTrials.gov, NCT04744532, as Induced pluripotent stem cell-based Drug Repurposing for Amyotrophic Lateral Sclerosis Medicine (iDReAM) study.
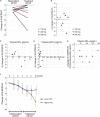
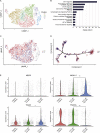
Findings: Between March 29, 2019 and May 7, 2021, 20 patients were enrolled, 13 of whom received bosutinib treatment and 12 were included in the safety and efficacy analyses. No DLTs were observed up to 300 mg QD, but DLTs were observed in 3/3 patients of the 400 mg QD cohort. In all patients receiving 100 mg-400 mg, the prevalent adverse events (AEs) were gastrointestinal AEs in 12 patients (92.3%), liver function related AEs in 7 patients (53.8%), and rash in 3 patients (23.1%). The safety profile was consistent with that known for CML treatment, and ALS-specific AEs were not observed. A subset of patients (5/9 patients) was found to respond well to bosutinib treatment over the 12-week treatment period. It was found that the treatment-responsive patients could be distinguished by their lower levels of plasma NFL. Furthermore, single-cell RNA sequencing of iPSC-derived motor neurons revealed the pathogenesis related molecular signature in patients with ALS showing responsiveness to bosutinib.
Interpretation: This is the first trial of a Src/c-Abl inhibitor, bosutinib, for patients with ALS. The safety and tolerability of bosutinib up to 300 mg, not 400 mg, in ALS were described, and responsiveness of patients on motor function was observed. Since this was an open-label trial within a short period with a limited number of patients, further clinical trials will be required.
Funding: AMED and iPS Cell Research Fund.
© 2022 The Author(s).
Conflict of interest statement
RU reports consulting fees from Eisai, Sawai Pharmaceutical, and EP Croit. NT reports speakers bureaus fees from Pfizer, Novartis Pharmaceuticals, and Otsuka Pharmaceutical. RT reports consulting fees from Kan Institute. RT and NT received research funding and honoraria for lectures from Pfizer. The other authors declare that they have no competing interests.
Figures

References
-
- Maurel C., Dangoumau A., Marouillat S., et al. Causative genes in amyotrophic lateral sclerosis and protein degradation pathways: a link to neurodegeneration. Mol Neurobiol. 2018;55(8):6480–6499. - PubMed
-
- Bensimon G., Lacomblez L., Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585–591. - PubMed
-
- Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16(7):505–512. - PubMed
-
- Miller T., Cudkowicz M., Shaw P.J., et al. Phase 1-2 trial of antisense oligonucleotide tofersen for SOD1 ALS. N Engl J Med. 2020;383(2):109–119. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

