Preservation of antigen-specific responses in cryopreserved CD4+ and CD8+ T cells expanded with IL-2 and IL-7
- PMID: 36467614
- PMCID: PMC9713293
- DOI: 10.1016/j.jtauto.2022.100173
Preservation of antigen-specific responses in cryopreserved CD4+ and CD8+ T cells expanded with IL-2 and IL-7
Abstract
Objectives: We sought to develop medium throughput standard operating procedures for screening cryopreserved human peripheral blood mononuclear cells (PBMCs) for CD4+ and CD8+ T cell responses to potential autoantigens.
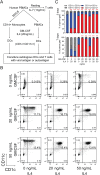
Methods: Dendritic cells were loaded with a peptide cocktail from ubiquitous viruses or full-length viral protein antigens and cocultured with autologous T cells. We measured expression of surface activation markers on T cells by flow cytometry and cytometry by time of flight 24-72 h later. We tested responses among T cells freshly isolated from healthy control PBMCs, cryopreserved T cells, and T cells derived from a variety of T cell expansion protocols. We also compared the transcriptional profile of CD8+ T cells rested with interleukin (IL)7 for 48 h after 1) initial thawing, 2) expansion, and 3) secondary cryopreservation/thawing of expanded cells. To generate competent antigen presenting cells from PBMCs, we promoted differentiation of PBMCs into dendritic cells with granulocyte macrophage colony stimulating factor and IL-4.
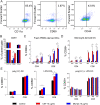
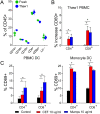
Results: We observed robust dendritic cell differentiation from human PBMCs treated with 50 ng/mL GM-CSF and 20 ng/mL IL-4 in as little as 3 days. Dendritic cell purity was substantially increased by magnetically enriching for CD14+ monocytes prior to differentiation. We also measured antigen-dependent T cell activation in DC-T cell cocultures. However, polyclonal expansion of T cells with anti-CD3/antiCD28 abolished antigen-dependent upregulation of CD69 in our assay despite minimal transcriptional differences between rested CD8+ T cells before and after expansion. Furthermore, resting these expanded T cells in IL-2, IL-7 or IL-15 did not restore the antigen dependent responses. In contrast, T cells that were initially expanded with IL-2 + IL-7 rather than plate bound anti-CD3 + anti-CD28 retained responsiveness to antigen stimulation and these responses strongly correlated with responses measured at initial thawing.
Significance: While screening techniques for potential pathological autoantibodies have come a long way, comparable full-length protein target assays for screening patient T cells at medium throughput are noticeably lacking due to technical hurdles. Here we advance techniques that should have broad applicability to translational studies investigating cell mediated immunity in infectious or autoimmune diseases. Future studies are aimed at investigating possible CD8+ T cell autoantigens in MS and other CNS autoimmune diseases.
Keywords: Antigen stimulation; Dendritic cells; Monocytes; Polyclonal expansion; T cell.
© 2022 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Spira A., Yurgelun M.B., Alexandrov L., Rao A., Bejar R., Polyak K., Giannakis M., Shilatifard A., Finn O.J., Dhodapkar M., Kay N.E., Braggio E., Vilar E., Mazzilli S.A., Rebbeck T.R., Garber J.E., Velculescu V.E., Disis M.L., Wallace D.C., Lippman S.M. Precancer atlas to drive precision prevention trials. Cancer Res. 2017;77:1510–1541. - PMC - PubMed
-
- Spira A., Disis M.L., Schiller J.T., Vilar E., Rebbeck T.R., Bejar R., Ideker T., Arts J., Yurgelun M.B., Mesirov J.P., Rao A., Garber J., Jaffee E.M., Lippman S.M. Leveraging premalignant biology for immune-based cancer prevention. Proc. Natl. Acad. Sci. U. S. A. 2016;113:10750–10758. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

