Setting up of a hospital Covid-19 vaccination center: A descriptive study
- PMID: 36467756
- PMCID: PMC9713314
- DOI: 10.1002/hsr2.968
Setting up of a hospital Covid-19 vaccination center: A descriptive study
Abstract
Background and aims: The coronavirus pandemic challenged countries worldwide in a race against contaminations and variants. Vaccination campaigns were the answer to such an infectious spread. This descriptive study presents the organizational process of the setting up of a Covid-19 vaccination center in a French University Hospital in January 2021, the issues encountered along the way and assessment of adaptability.
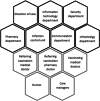
Methods: Three major stakeholders: SARS CoV-2 crisis referent, referring vaccination medical doctor and referring vaccination pharmacist retraced key moments and identified issues encountered during the setting up of the vaccination center and its long term maintenance, threw a series of meetings. Records of crisis and periodic meetings that took place threw out the vaccination campaign were consulted.
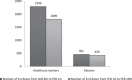
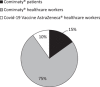
Results: A multidisciplinary crisis steering committee with nine different professionals was created January 3. Logistics for the vaccination center opening were discussed: location, informatics, appointment-scheduling, pharmaceutical circuit, internal circuit, human resources, and information communication. The vaccination center was ready to welcome healthcare workers in less than 24 h on January 4. The first month, 2757 1st shots were administered, leading up to a total of 9167 1st shots during 6 months of activity. From January to June 2021, the multidisciplinary group dealt and adapted its processes to challenging and unexpected situations. Indeed, issues encountered with Pfizer BioNTech's and AstraZeneca's vaccine, were: supply shortages, vaccine manipulation, targeted populations, pharmacovigilance, and general communication.
Conclusion: This descriptive study provides an exclusive insight on how a hospital vaccination center was organized and adapted during Covid-19 pandemic to ensure healthcare workers' security and resilience, and to protect high risk patients of severe Covid-19 infection.
Keywords: Covid‐19 pandemic; hospital vaccination campaign; management; multidisciplinary cooperation; organization; public health.
© 2022 The Authors. Health Science Reports published by Wiley Periodicals LLC.
Conflict of interest statement
The author declares no conflict of interest.
Figures
References
-
- EMA recommends first COVID‐19 vaccine for authorisation in the EU . European Medicines Agency. [Online] December 21, 2020. Accessed December 21, 2020. https://www.ema.europa.eu/en/news/ema-recommends-first-covid-19-vaccine-...
-
- Stratégie de vaccination contre le Sars‐Cov‐2—Recommandations préliminaires sur la stratégie de priorisation des populations à vacciner . Haute Autorité de Santé. [Online] November 30, 2020. https://www.has-sante.fr/jcms/p_3221338/fr/strategie-de-vaccination-cont...
-
- Fournier S, Godeau B, Lucet J‐C, et al. Organisation du parcours des patients, de la protection des patients et des personnels hospitaliers à l'heure du confinement et de la reprise d'activité non COVID‐19. Assistance Publique‐Hopitaux de Paris. 2020. Published online April 20, 2020. Accessed April 26, 2021. https://aphp.aphp.fr/wp-content/blogs.dir/268/files/2020/04/Parcours-Pro...
-
- Comirnaty EPAR product information . European Medicines Agency. [Online] December 21, 2020, Accessed December 21, 2020. https://www.ema.europa.eu/en/documents/product-information/comirnaty-epa...
LinkOut - more resources
Full Text Sources
Miscellaneous