Managing relapsed refractory lymphoma with palliative oral chemotherapy: A multicentre retrospective study
- PMID: 36467809
- PMCID: PMC9713053
- DOI: 10.1002/jha2.537
Managing relapsed refractory lymphoma with palliative oral chemotherapy: A multicentre retrospective study
Abstract
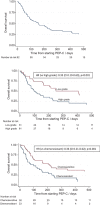
PEP-C (prednisolone, etoposide, procarbazine and cyclophosphamide) is an orally administered daily chemotherapy regimen used with palliative intent in relapsed refractory lymphoma. To our knowledge, no data on PEP-C have been reported since the original group described the regimen. Here we present a multicentre retrospective cohort reporting our use of PEP-C in 92 patients over an 8-year period. We find that even heavily pretreated lymphoma can respond to PEP-C, particularly low-grade lymphoma (including mantle cell) and lymphoma that was sensitive to the previous line of systemic therapy (chemosensitive). These characteristics may help in the selection of patients likely to derive benefit. The median overall survival of patients with chemosensitive lymphoma treated with PEP-C is 217 days. Within the limitations of a retrospective cohort, we find that PEP-C is well tolerated: the most common toxicity leading to discontinuation is marrow suppression. We suggest that PEP-C should be considered for patients with relapsed refractory lymphoma in two settings: first, where there is no licensed alternative; and second, where the licensed alternative is an intravenous drug and the patient would prefer to choose an oral chemotherapy option.
© 2022 The Authors. eJHaem published by British Society for Haematology and John Wiley & Sons Ltd.
Figures
References
-
- Salles G, Duell J, González Barca E, Tournilhac O, Jurczak W, Liberati AM, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B‐cell lymphoma (L‐MIND): a multicentre, prospective, single‐arm, phase 2 study. Lancet Oncol. 2020;21(7):978–88. - PubMed
-
- Pettengell R, Coiffier B, Narayanan G, de Mendoza FH, Digumarti R, Gomez H, et al. Pixantrone dimaleate versus other chemotherapeutic agents as a single‐agent salvage treatment in patients with relapsed or refractory aggressive non‐Hodgkin lymphoma: a phase 3, multicentre, open‐label, randomised trial. Lancet Oncol. 2012;13(7):696–706. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
