Stability Estimation of Gallium Complexes of DOTA Derivatives for Radiotheranostic Applications
- PMID: 36467905
- PMCID: PMC9713862
- DOI: 10.1021/acsomega.2c06814
Stability Estimation of Gallium Complexes of DOTA Derivatives for Radiotheranostic Applications
Abstract
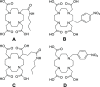
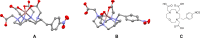
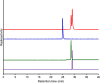
1,4,7,10-Tetraazacyclododecane-1,4,7-triacetic acid (DO3A) has been used to prepare 68Ga-labeled probes for the diagnostic counterpart of radiotheranostic applications. While DO3A provides stable complexes with therapeutic radionuclides such as 90Y, 177Lu, and 225Ac, further improvement of the in vivo stability of the Ga-DO3A complex is required. Considering the high stability of an intact Ga-DOTA complex, the stability of Ga complexes of DOTA and DO3A derivatives, including benzyl-DOTA (Bn-DOTA), was evaluated to gain fundamental knowledge for developing the next-generation radiotheranostic probes using 68Ga as a diagnostic counterpart. Following the complexation reaction to prepare 67Ga-labeled DOTA and DO3A derivatives, the stability of the resulting 67Ga-labeled compounds was evaluated in murine plasma and apo-transferrin challenge. [67Ga]Ga-Bn-DOTA produced two isomers, and one of the isomers exhibited the highest stability among the tested complexes. The X-ray crystallography showed that the less stable isomer of Ga-Bn-DOTA suggested an N3O3 coordination geometry, while Ga-DOTA and Ga-Bn-DO3A show N4O2 coordination. To further evaluate the stability, a synthetic somatostatin analogue, [Tyr3]octreotide (TOC), was used as a model peptide, and p-COOH-Bn-DOTA and DO3A were conjugated with TOC to prepare DOTA-Bn-TOC and DOTATOC. [67Ga]Ga-DOTA-Bn-TOC also yielded two isomers with varying stability, and one isomer exhibited significantly higher stability than [67Ga]Ga-DOTATOC both in vitro and in vivo. These findings indicate that para-substituted Bn-DOTA would constitute a suitable chelating agent for developing next-generation radiotheranostic probes, although high-performance liquid chromatography purification is needed. Thus, further chemical modification on the Bn-DOTA molecule is also needed to avoid the formation of a Ga complex with the N3O3 configuration.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Clarke E. T.; Martell A. E. Stabilities of trivalent metal ion complexes of the tetraacetate derivatives of 12-, 13- and 14-membered tetraazamacrocycles. Inorg. Chim. Acta 1991, 190, 37–46. 10.1016/s0020-1693(00)80229-7. - DOI
-
- Kumar K.; Chang C. A.; Francesconi L.; Dischino D.; Malley M.; Gougoutas J.; Tweedle M. F. Synthesis, Stability, and Structure of Gadolinium(III) and Yttrium(III) Macrocyclic Poly(amino carboxylates). Inorg. Chem. 1994, 33, 3567–3575. 10.1021/ic00094a021. - DOI
-
- Tóth Ė.; Brücher E. Stability constants of the lanthanide (III)-1,4,7,10-tetraazacyclododecane-N,N’,N’’,N’’’-tetraacetate complexes. Inorg. Chim. Acta 1994, 221, 165–167. 10.1016/0020-1693(94)03964-X. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

