Reevaluation of a Bicyclic Pyrazoline as a Selective 15-Lipoxygenase V-Type Activator Possessing Fatty Acid Specificity
- PMID: 36467910
- PMCID: PMC9713885
- DOI: 10.1021/acsomega.2c05877
Reevaluation of a Bicyclic Pyrazoline as a Selective 15-Lipoxygenase V-Type Activator Possessing Fatty Acid Specificity
Abstract
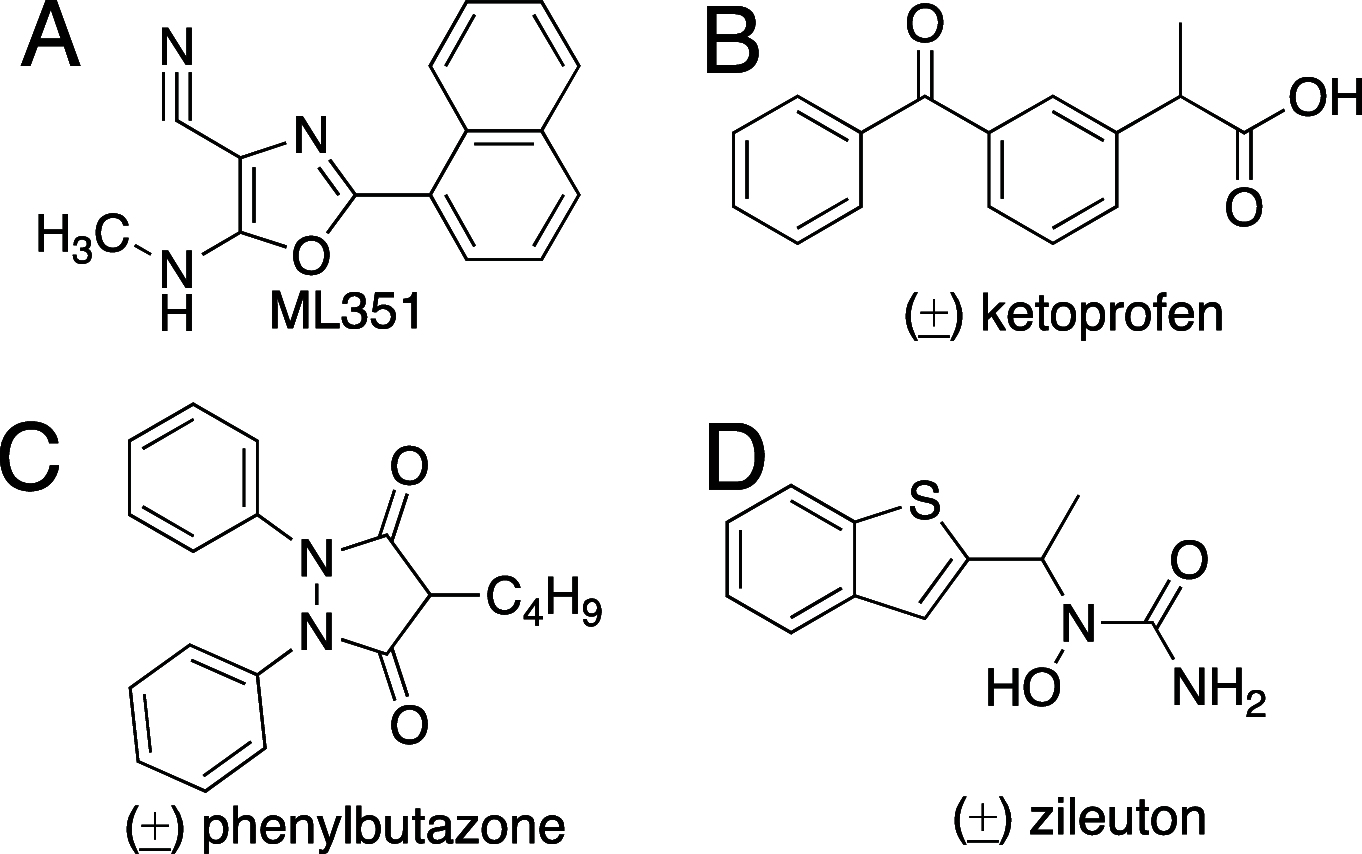
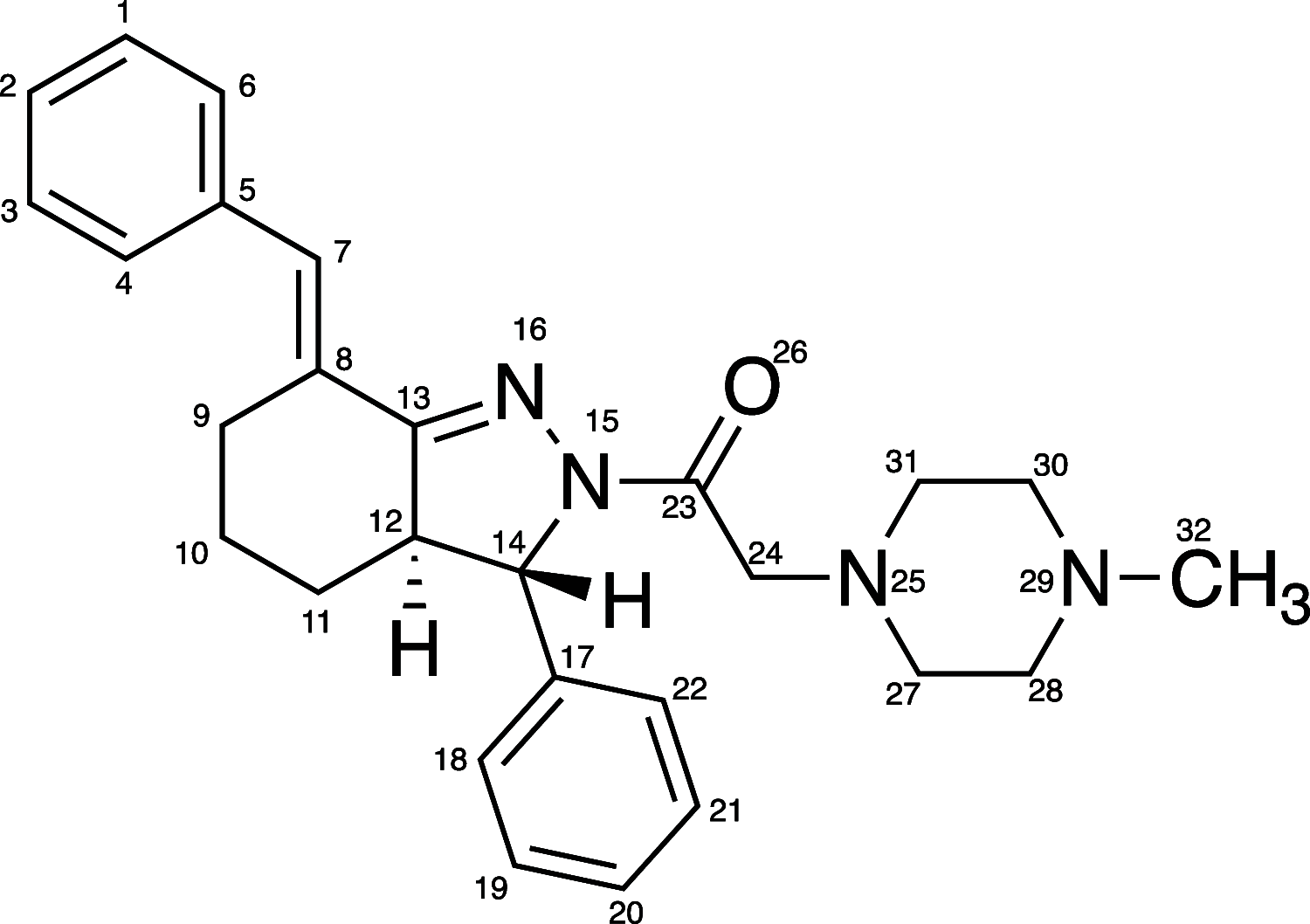
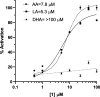
Regulation of lipoxygenase (LOX) activity is of great interest due to the involvement of the various LOX isoforms in the inflammatory process and hence many diseases. The bulk of investigations have centered around the discovery and design of inhibitors. However, the emerging understanding of the role of h15-LOX-1 in the resolution of inflammation provides a rationale for the development of activators as well. Bicyclic pyrazolines are known bioactive molecules that have been shown to display antibiotic and anti-inflammatory activities. In the current work, we reevaluated a previously discovered bicyclic pyrazoline h15-LOX-1 activator, PKUMDL_MH_1001 (written as 1 for this publication), and determined that it is inactive against other human LOX isozymes, h5-LOX, h12-LOX, and h15-LOX-2. Analytical characterization of 1 obtained in the final synthesis step identified it as a mixture of cis- and trans-diastereomers: cis-1 (12%) and trans-1 (88%); and kinetic analysis indicated similar potency between the two. Using compound 1 as the cis-trans mixture, h15-LOX-1 catalysis with arachidonic acid (AA) (AC50 = 7.8 +/- 1 μM, A max = 240%) and linoleic acid (AC50 = 5.3 +/- 0.7 μM, A max = 98%) was activated, but not with docosahexaenoic acid (DHA) or mono-oxylipins. Steady-state kinetics demonstrate V-type activation for 1, with a β value of 2.2 +/- 0.4 and an K x of 16 +/- 1 μM. Finally, it is demonstrated that the mechanism of activation for 1 is likely not due to decreasing substrate inhibition, as was postulated previously. 1 also did not affect the activity of the h15-LOX-1 selective inhibitor, ML351, nor did 1 affect the activity of allosteric effectors, such as 12S-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (12S-HETE) and 14S-hydroperoxy-4Z,7Z,10Z,12E,16Z,19Z-docosahexaenoic acid (14S-HpDHA). These data confirm that 1 binds to a distinct activation binding site, as previously postulated. Future work should be aimed at the development of selective activators that are capable of activating h15-LOX-1 catalysis with DHA, thus enhancing the production of DHA-derived pro-resolution biomolecules.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Biosynthesis of the Maresin Intermediate, 13S,14S-Epoxy-DHA, by Human 15-Lipoxygenase and 12-Lipoxygenase and Its Regulation through Negative Allosteric Modulators.Biochemistry. 2020 May 19;59(19):1832-1844. doi: 10.1021/acs.biochem.0c00233. Epub 2020 May 7. Biochemistry. 2020. PMID: 32324389 Free PMC article.
-
In Vitro Biosynthetic Pathway Investigations of Neuroprotectin D1 (NPD1) and Protectin DX (PDX) by Human 12-Lipoxygenase, 15-Lipoxygenase-1, and 15-Lipoxygenase-2.Biochemistry. 2021 Jun 8;60(22):1741-1754. doi: 10.1021/acs.biochem.0c00931. Epub 2021 May 24. Biochemistry. 2021. PMID: 34029049 Free PMC article.
-
15-Lipoxygenase-1 biosynthesis of 7S,14S-diHDHA implicates 15-lipoxygenase-2 in biosynthesis of resolvin D5.J Lipid Res. 2020 Jul;61(7):1087-1103. doi: 10.1194/jlr.RA120000777. Epub 2020 May 13. J Lipid Res. 2020. PMID: 32404334 Free PMC article.
-
The eosinophil chemoattractant 5-oxo-ETE and the OXE receptor.Prog Lipid Res. 2013 Oct;52(4):651-65. doi: 10.1016/j.plipres.2013.09.001. Epub 2013 Sep 19. Prog Lipid Res. 2013. PMID: 24056189 Free PMC article. Review.
-
Structure, biochemistry and biology of hepoxilins: an update.FEBS J. 2007 Jul;274(14):3503-3512. doi: 10.1111/j.1742-4658.2007.05910.x. Epub 2007 Jul 2. FEBS J. 2007. PMID: 17608719 Review.
Cited by
-
Structural and Functional Biology of Mammalian ALOX Isoforms with Particular Emphasis on Enzyme Dimerization and Their Allosteric Properties.Int J Mol Sci. 2024 Nov 9;25(22):12058. doi: 10.3390/ijms252212058. Int J Mol Sci. 2024. PMID: 39596127 Free PMC article. Review.
-
Identification of lipid senolytics targeting senescent cells through ferroptosis induction.bioRxiv [Preprint]. 2024 Oct 14:2024.10.14.618023. doi: 10.1101/2024.10.14.618023. bioRxiv. 2024. PMID: 39463954 Free PMC article. Preprint.
References
-
- Shrivastava A.; Chakraborty A. K.; Upmanyu N.; Singh A. Recent Progress in Chemistry and Biology of Indazole and its Derivatives: a Breif Reiew. Austin J. Anal. Pharm. Chem. 2016, 3, 1076.
-
- Elie J.; Vercouillie J.; Arlicot N.; Lemaire L.; Bidault R.; Bodard S.; Hosselet C.; Deloye J. B.; Chalon S.; Emond P.; Guilloteau D.; Buron F.; Routier S. Design of selective COX-2 inhibitors in the (aza)indazole series. Chemistry, in vitro studies, radiochemistry and evaluations in rats of a [(18)F] PET tracer. J. Enzyme Inhib. Med. Chem. 2019, 34, 1–7. 10.1080/14756366.2018.1501043. - DOI - PMC - PubMed
-
- Polo-Cuadrado E.; Acosta-Quiroga K.; Rojas-Pena C.; Rodriguez-Nunez Y. A.; Duarte Y.; Brito I.; Cisterna J.; Gutierrez M. Molecular modeling and structural analysis of some tetrahydroindazole and cyclopentanepyrazole derivatives as COX-2 inhibitors. Arabian J. Chem. 2022, 15, 1–13. 10.1016/j.arabjc.2021.103540. - DOI
-
- Minu M.; Singh D.; Mahaddalkar T.; Lopus M.; Winter P.; Ayoub A. T.; Missiaen K.; Tilli T. M.; Pasdar M.; Tuszynski J. Chemical synthesis, pharmacological evaluation and in silico analysis of new 2,3,3a,4,5,6-hexahydrocyclopenta[c]pyrazole derivatives as potential anti-mitotic agents. Bioorg. Med. Chem. Lett. 2016, 26, 3855–3861. 10.1016/j.bmcl.2016.07.025. - DOI - PubMed

