Establishment of Quality Evaluation Method for Yinqiao Powder: A Herbal Formula against COVID-19 in China
- PMID: 36467981
- PMCID: PMC9718632
- DOI: 10.1155/2022/1748324
Establishment of Quality Evaluation Method for Yinqiao Powder: A Herbal Formula against COVID-19 in China
Abstract
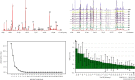
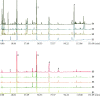
Yinqiao powder, with significant anti-inflammatory and antiviral effects, is a classical formula for the treatment of febrile diseases in China. During the SARS period in 2003, Yinqiao powder showed a good antipyretic effect. It also plays a major role in the treatment for COVID-19 in China. Although there are many studies on the chemical compositions and pharmacological effects of Yinqiao powder, there are few studies on the quality standard system of it. In our study, a systematic quality evaluation method of Yinqiao powder combining HPLC fingerprint with quantitative analysis of multi-components by single marker (QAMS) based on network pharmacology and UPLC-Q-Exactive-Orbitrap-MS was established for the first time. In the UPLC-Q-Exactive-Orbitrap-MS experiment, a total of 53 compounds were identified in the extract solution of Yinqiao powder. In addition, 33 blood components were characterized, 23 of which were prototypes. The results of network pharmacology analysis showed that Yinqiao powder may inhibit inflammatory responses by suppressing IL-6, CXCL2, TNFα, NF-κB, etc., in the treatment of COVID-19. The HPLC fingerprint analysis of Yinqiao powder was conducted at 237 nm and 29 characteristic peaks were matched, 11 of which were identified. Forsythoside A was selected as the internal standard reference and double-wavelength (237 nm and 327 nm) was established in QAMS experiment. The repeatability was well under different conditions, and the results measured by QAMS were consisted with that of the external standard method (ESM), indicating that the QAMS method was reliable and accurate. The quality evaluation method of Yinqiao powder would be helpful to evaluate the intrinsic quality of Yinqiao powder more comprehensively, which is conducive to improve the quality standard of Yinqiao powder and provide a beneficial guarantee for the clinical treatment of COVID-19.
Copyright © 2022 Huimin Zhang et al.
Conflict of interest statement
Author Aijun Zhang is employed by Shandong Hongjitang Pharmaceutical Group Co., Ltd. The remaining authors declare that they have no conflicts of interest.
Figures

Similar articles
-
[Comparative analysis of powder and piece decocting processes of Yinqiao Powder based on determination of multiple primary components].Zhongguo Zhong Yao Za Zhi. 2022 Jul;47(14):3788-3797. doi: 10.19540/j.cnki.cjcmm.20210913.302. Zhongguo Zhong Yao Za Zhi. 2022. PMID: 35850836 Chinese.
-
Rapid identification of chemical constituents in Yinqiao powder using ultra-high-performance liquid chromatography coupled to quadrupole-time-of-flight tandem mass spectrometry using data filtering strategy.Biomed Chromatogr. 2022 Aug;36(8):e5392. doi: 10.1002/bmc.5392. Epub 2022 May 11. Biomed Chromatogr. 2022. PMID: 35491476
-
[Chemical pattern recognition of Atractylodes chinensis from different producing areas and establishment of quantitative analysis of multi-components by single marker (QAMS) method for four components].Zhongguo Zhong Yao Za Zhi. 2022 Aug;47(16):4395-4402. doi: 10.19540/j.cnki.cjcmm.20211217.201. Zhongguo Zhong Yao Za Zhi. 2022. PMID: 36046868 Chinese.
-
[UPLC fingerprint combined with quantitative analysis of multi-components by single marker for quality assessment of Danshen Injection].Zhongguo Zhong Yao Za Zhi. 2019 Sep;44(17):3724-3731. doi: 10.19540/j.cnki.cjcmm.20190410.310. Zhongguo Zhong Yao Za Zhi. 2019. PMID: 31602945 Chinese.
-
[Changes of specific chromatograms and contents of five components in Yinqiao powder decoctions during decocting process].Zhongguo Zhong Yao Za Zhi. 2016 Jan;41(1):60-64. doi: 10.4268/cjcmm20160112. Zhongguo Zhong Yao Za Zhi. 2016. PMID: 28845641 Chinese.
Cited by
-
[Comparison of anti-inflammatory, antibacterial and analgesic activities of formulated granules versus traditional decoction of Yinqiao Powder].Nan Fang Yi Ke Da Xue Xue Bao. 2025 May 20;45(5):1003-1012. doi: 10.12122/j.issn.1673-4254.2025.05.13. Nan Fang Yi Ke Da Xue Xue Bao. 2025. PMID: 40415432 Free PMC article. Chinese.
-
Traditional Chinese Medicine for Viral Pneumonia Therapy: Pharmacological Basis and Mechanistic Insights.Int J Biol Sci. 2025 Jan 6;21(3):989-1013. doi: 10.7150/ijbs.105086. eCollection 2025. Int J Biol Sci. 2025. PMID: 39897040 Free PMC article. Review.
-
Molecular mechanism of honeysuckle + forsythia in treatment of acute lung injury based on network pharmacology.Biomed Rep. 2024 Jan 3;20(2):32. doi: 10.3892/br.2024.1720. eCollection 2024 Feb. Biomed Rep. 2024. PMID: 38273899 Free PMC article.
-
The Role of Yinqiao Powder in Modulating Pseudomonas aeruginosa Biofilm and Virulence Factors.Infect Drug Resist. 2025 Mar 12;18:1405-1414. doi: 10.2147/IDR.S507257. eCollection 2025. Infect Drug Resist. 2025. PMID: 40098712 Free PMC article.
References
-
- Li D. X. A clinical analysis of treating children’s HFMD with the Zhuye Shigao decoction. Clinical Journal of Chinese Medicine . 2017;9:63–65.
-
- Guo R., Zhao M., Liu H., et al. Uncovering the pharmacological mechanisms of Xijiao Dihuang decoction combined with Yinqiao powder in treating influenza viral pneumonia by an integrative pharmacology strategy. Biomedicine & Pharmacotherapy . 2021;141 doi: 10.1016/j.biopha.2021.111676.111676 - DOI - PubMed
-
- Liu Y. L., Lin L. F., Luo Y. T., Wu Y. Q., Li H. Research progress of heat-clearing and detoxifying traditional Chinese medicine in treatment of corona virus disease 2019. Chinese archives of traditional Chinese medicine . 2021;39:181–186.
LinkOut - more resources
Full Text Sources
Miscellaneous

