Red cell exchange transfusions increase cerebral capillary transit times and may alter oxygen extraction in sickle cell disease
- PMID: 36468659
- PMCID: PMC10106384
- DOI: 10.1002/nbm.4889
Red cell exchange transfusions increase cerebral capillary transit times and may alter oxygen extraction in sickle cell disease
Abstract
Persons with sickle cell disease (SCD) suffer from chronic hemolytic anemia, reduced blood oxygen content, and lifelong risk of silent and overt stroke. Major conventional stroke risk factors are absent in most individuals with SCD, yet nearly 50% have evidence of brain infarcts by the age of 30 years, indicating alternative etiologies for ischemia. We investigated whether radiological evidence of accelerated blood water transit through capillaries, visible on arterial spin labeling (ASL) magnetic resonance imaging, reduces following transfusion-induced increases in hemoglobin and relates to oxygen extraction fraction (OEF). Neurological evaluation along with anatomical and hemodynamic imaging with cerebral blood flow (CBF)-weighted pseudocontinuous ASL and OEF imaging with T2 -relaxation-under-spin-tagging were applied in sequence before and after blood transfusion therapy (n = 32) and in a comparator cohort of nontransfused SCD participants on hydroxyurea therapy scanned at two time points to assess stability without interim intervention (n = 13). OEF was calculated separately using models derived from human hemoglobin-F, hemoglobin-A, and hemoglobin-S. Gray matter CBF and dural sinus signal, indicative of rapid blood transit, were evaluated at each time point and compared with OEF using paired statistical tests (significance: two-sided p < 0.05). No significant change in sinus signal was observed in nontransfused participants (p = 0.650), but a reduction was observed in transfused participants (p = 0.034), consistent with slower red cell transit following transfusion. The dural sinus signal intensity was inversely associated with OEF pretransfusion (p = 0.011), but not posttransfusion. Study findings suggest that transfusion-induced increases in total hemoglobin may lengthen blood transit times through cerebral capillaries and alter cerebral OEF in SCD.
Keywords: capillary; cerebral blood flow; oxygen extraction fraction; shunting; sickle cell disease; stroke.
© 2022 John Wiley & Sons Ltd.
Figures

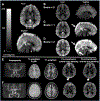


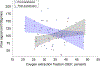
References
-
- Adams RJ, McKie VC, Hsu L, Files B, Vichinsky E, Pegelow C, Abboud M, Gallagher D, Kutlar A, Nichols FT, Bonds DR, Brambilla D. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. The New England journal of medicine. 1998;339(1):5–11. doi: 10.1056/NEJM199807023390102. - DOI - PubMed
-
- DeBaun MR, Gordon M, McKinstry RC, Noetzel MJ, White DA, Sarnaik SA, Meier ER, Howard TH, Majumdar S, Inusa BP, Telfer PT, Kirby-Allen M, McCavit TL, Kamdem A, Airewele G, Woods GM, Berman B, Panepinto JA, Fuh BR, Kwiatkowski JL, King AA, Fixler JM, Rhodes MM, Thompson AA, Heiny ME, Redding-Lallinger RC, Kirkham FJ, Dixon N, Gonzalez CE, Kalinyak KA, Quinn CT, Strouse JJ, Miller JP, Lehmann H, Kraut MA, Ball WS Jr., Hirtz D, Casella JF. Controlled trial of transfusions for silent cerebral infarcts in sickle cell anemia. The New England journal of medicine. 2014;371(8):699–710. Epub 2014/08/21. doi: 10.1056/NEJMoa1401731. - DOI - PMC - PubMed
-
- Juttukonda MR, Lee CA, Patel NJ, Davis LT, Waddle SL, Gindville MC, Pruthi S, Kassim AA, DeBaun MR, Donahue MJ, Jordan LC. Differential cerebral hemometabolic responses to blood transfusions in adults and children with sickle cell anemia. Journal of magnetic resonance imaging : JMRI. 2019;49(2):466–77. Epub 2018/10/17. doi: 10.1002/jmri.26213. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

