Glycine inhibits NINJ1 membrane clustering to suppress plasma membrane rupture in cell death
- PMID: 36468682
- PMCID: PMC9754625
- DOI: 10.7554/eLife.78609
Glycine inhibits NINJ1 membrane clustering to suppress plasma membrane rupture in cell death
Abstract
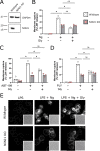
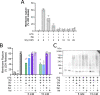
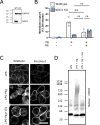
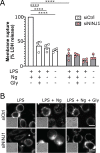
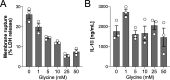
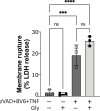
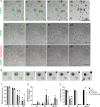
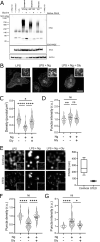
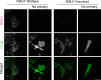
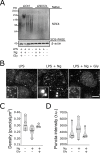
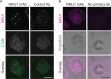
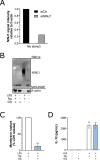
First recognized more than 30 years ago, glycine protects cells against rupture from diverse types of injury. This robust and widely observed effect has been speculated to target a late downstream process common to multiple modes of tissue injury. The molecular target of glycine that mediates cytoprotection, however, remains elusive. Here, we show that glycine works at the level of NINJ1, a newly identified executioner of plasma membrane rupture in pyroptosis, necrosis, and post-apoptosis lysis. NINJ1 is thought to cluster within the plasma membrane to cause cell rupture. We demonstrate that the execution of pyroptotic cell rupture is similar for human and mouse NINJ1 and that NINJ1 knockout functionally and morphologically phenocopies glycine cytoprotection in macrophages undergoing lytic cell death. Next, we show that glycine prevents NINJ1 clustering by either direct or indirect mechanisms. In pyroptosis, glycine preserves cellular integrity but does not affect upstream inflammasome activities or accompanying energetic cell death. By positioning NINJ1 clustering as a glycine target, our data resolve a long-standing mechanism for glycine-mediated cytoprotection. This new understanding will inform the development of cell preservation strategies to counter pathologic lytic cell death.
Keywords: NINJ1; cell biology; cytoprotection; glycine; human; immunology; inflammation; macrophages; mouse; necrosis; pyroptosis.
© 2022, Borges, Sætra et al.
Conflict of interest statement
JB, RS, AV, MB, PD, BS, BK, CE, JK, NG, TF, BS No competing interests declared
Figures


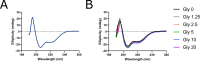
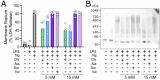
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

