Biosynthetic neoantigen displayed on bacteria derived vesicles elicit systemic antitumour immunity
- PMID: 36468941
- PMCID: PMC9721206
- DOI: 10.1002/jev2.12289
Biosynthetic neoantigen displayed on bacteria derived vesicles elicit systemic antitumour immunity
Abstract
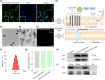
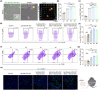
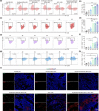
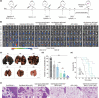
Neoantigens derived from mutant proteins in tumour cells could elicit potent personalized anti-tumour immunity. Nevertheless, the layout of vaccine vehicle and synthesis of neoantigen are pivotal for stimulating robust response. The power of synthetic biology enables genetic programming bacteria to produce therapeutic agents under contol of the gene circuits. Herein, we genetically engineered bacteria to synthesize fusion neoantigens, and prepared bacteria derived vesicles (BDVs) presenting the neoantigens (BDVs-Neo) as personalized therapeutic vaccine to drive systemic antitumour response. BDVs-Neo and granulocyte-macrophage colony-stimulating factor (GM-CSF) were inoculated subcutaneously within hydrogel (Gel), whereas sustaining release of BDVs-Lipopolysaccharide (LPS) and GM-CSF recruited the dendritic cells (DCs). Virtually, Gel-BDVs-Neo combined with the programmed cell death protein 1 (PD-1) antibody intensively enhanced proliferation and activation of tumour-infiltrated T cells, as well as memory T cell clone expansion. Consequently, BDVs-Neo combining with checkpoint blockade therapy effectively prevented tumour relapse and metastasis.
Keywords: bacteria derived vesicles (BDVs); cancer vaccines; checkpoint blockade; immunotherapy; neoantigen.
© 2022 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures


Similar articles
-
Neoantigen sequestrated autophagosomes as therapeutic cancer vaccines.J Control Release. 2024 Dec;376:369-381. doi: 10.1016/j.jconrel.2024.10.019. Epub 2024 Oct 19. J Control Release. 2024. PMID: 39413847
-
Therapy of established tumour with a hybrid cellular vaccine generated by using granulocyte-macrophage colony-stimulating factor genetically modified dendritic cells.Immunology. 1999 Aug;97(4):616-25. doi: 10.1046/j.1365-2567.1999.00823.x. Immunology. 1999. PMID: 10457215 Free PMC article.
-
The boosting effect of co-transduction with cytokine genes on cancer vaccine therapy using genetically modified dendritic cells expressing tumor-associated antigen.Int J Oncol. 2006 Apr;28(4):947-53. Int J Oncol. 2006. PMID: 16525645
-
Biomaterials-assisted construction of neoantigen vaccines for personalized cancer immunotherapy.Expert Opin Drug Deliv. 2023 Mar;20(3):323-333. doi: 10.1080/17425247.2023.2168640. Epub 2023 Jan 19. Expert Opin Drug Deliv. 2023. PMID: 36634017 Review.
-
Engineering neoantigen vaccines to improve cancer personalized immunotherapy.Int J Biol Sci. 2022 Sep 1;18(15):5607-5623. doi: 10.7150/ijbs.76281. eCollection 2022. Int J Biol Sci. 2022. PMID: 36263174 Free PMC article. Review.
Cited by
-
A "CPApoptosis" nano-actuator switches immune-off solid tumors to immune-on for fueling T-cell- based immunotherapy.Theranostics. 2025 Mar 3;15(9):3797-3820. doi: 10.7150/thno.105867. eCollection 2025. Theranostics. 2025. PMID: 40213664 Free PMC article.
-
Exosomes, their sources, and possible uses in cancer therapy in the era of personalized medicine.J Cancer Res Clin Oncol. 2024 Dec 26;151(1):16. doi: 10.1007/s00432-024-06066-w. J Cancer Res Clin Oncol. 2024. PMID: 39724442 Free PMC article. Review.
-
Advances in immunotyping of colorectal cancer.Front Immunol. 2023 Oct 9;14:1259461. doi: 10.3389/fimmu.2023.1259461. eCollection 2023. Front Immunol. 2023. PMID: 37876934 Free PMC article. Review.
-
Ultrasound Responsive Nanovaccine Armed with Engineered Cancer Cell Membrane and RNA to Prevent Foreseeable Metastasis.Adv Sci (Weinh). 2023 Jul;10(19):e2301107. doi: 10.1002/advs.202301107. Epub 2023 Apr 25. Adv Sci (Weinh). 2023. PMID: 37097746 Free PMC article.
-
Roles of exosomes in immunotherapy for solid cancers.Cell Death Dis. 2024 Feb 1;15(2):106. doi: 10.1038/s41419-024-06494-z. Cell Death Dis. 2024. PMID: 38302430 Free PMC article. Review.
References
-
- Bachmann, M. F. , & Jennings, G. T. (2010). Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nature Reviews Immunology, 10, 787–796. - PubMed
-
- Banchereau, J. , & Palucka, A. K. (2005). Dendritic cells as therapeutic vaccines against cancer. Nature Reviews Immunology, 5, 296–306. - PubMed
-
- Cian, M. B. , Giordano, N. P. , Mettlach, J. A. , Minor, K. E. , & Dalebroux, Z. D. (2020). Separation of the cell envelope for gram‐negative bacteria into inner and outer membrane fractions with technical adjustments for Acinetobacter baumannii . Jove‐Journal of Visualized Experiments, 158, e60517. - PMC - PubMed
-
- Canale, F. P. , Basso, C. , Antonini, G. , Perotti, M. , Li, N. , Sokolovska, A. , Neumann, J. , James, M. J. , Geiger, S. , & Jin, W. J. (2021). Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature, 598, 662–666. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

