Tuberous sclerosis complex is associated with a novel human tauopathy
- PMID: 36469115
- PMCID: PMC10244026
- DOI: 10.1007/s00401-022-02521-5
Tuberous sclerosis complex is associated with a novel human tauopathy
Abstract
Tuberous sclerosis complex (TSC) is a neurogenetic disorder leading to epilepsy, developmental delay, and neurobehavioral dysfunction. The syndrome is caused by pathogenic variants in TSC1 (coding for hamartin) or TSC2 (coding for tuberin). Recently, we reported a progressive frontotemporal dementia-like clinical syndrome in a patient with a mutation in TSC1, but the neuropathological changes seen in adults with TSC with or without dementia have yet to be systematically explored. Here, we examined neuropathological findings in adults with TSC (n = 11) aged 30-58 years and compared them to age-matched patients with epilepsy unrelated to TSC (n = 9) and non-neurological controls (n = 10). In 3 of 11 subjects with TSC, we observed a neurofibrillary tangle-predominant "TSC tauopathy" not seen in epilepsy or non-neurological controls. This tauopathy was observed in the absence of pathological amyloid beta, TDP-43, or alpha-synuclein deposition. The neurofibrillary tangles in TSC tauopathy showed a unique pattern of post-translational modifications, with apparent differences between TSC1 and TSC2 mutation carriers. Tau acetylation (K274, K343) was prominent in both TSC1 and TSC2, whereas tau phosphorylation at a common phospho-epitope (S202) was observed only in TSC2. TSC tauopathy was observed in selected neocortical, limbic, subcortical, and brainstem sites and showed a 3-repeat greater than 4-repeat tau isoform pattern in both TSC1 and TSC2 mutation carriers, but no tangles were immunolabeled with MC1 or p62 antibodies. The findings suggest that individuals with TSC are at risk for a unique tauopathy in mid-life and that tauopathy pathogenesis may involve TSC1, TSC2, and related molecular pathways.
Keywords: Acetylation; Neurofibrillary tangle; TSC1; TSC2; Tau; Tauopathy; Tuberous sclerosis complex (TSC).
© 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
Figures

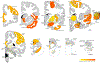


References
-
- Al-Saleem T, Wessner LL, Scheithauer BW, Patterson K, Roach ES, Dreyer SJ, Fujikawa K, Bjornsson J, Bernstein J, Henske EP (1998) Malignant tumors of the kidney, brain, and soft tissues in children and young adults with the tuberous sclerosis complex. Cancer 83: 2208–2216 - PubMed
-
- Alquezar C, Schoch KM, Geier EG, Ramos EM, Scrivo A, Li KH, Argouarch AR, Mlynarski EE, Dombroski B, DeTure M et al. (2021) TSC1 loss increases risk for tauopathy by inducing tau acetylation and preventing tau clearance via chaperone-mediated autophagy. Sci Adv 7: eabg3897 Doi 10.1126/sciadv.abg3897 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

