Signals of Adverse Drug Reactions Communicated by Pharmacovigilance Stakeholders: A Scoping Review of the Global Literature
- PMID: 36469249
- PMCID: PMC9883307
- DOI: 10.1007/s40264-022-01258-0
Signals of Adverse Drug Reactions Communicated by Pharmacovigilance Stakeholders: A Scoping Review of the Global Literature
Abstract
Introduction and objective: Signals of adverse drug reactions (ADRs) can be supported by reports of ADRs and by interventional and non-interventional studies. The evidence base and features of ADR reports that are used to support signals remain to be comprehensively described. To this end, we have undertaken a scoping review.
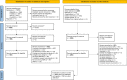
Methods: We searched the following databases: PubMed, EMBASE, PsycINFO, Web of Science, and Google Scholar, without language or time restrictions. We also hand searched the bibliographies of relevant studies. We included studies of any design if the results were described as signals. We assessed the levels of evidence using the Oxford Centre for Evidence-Based Medicine (OCEBM) criteria and coded features of reports of ADRs using the Bradford Hill guidelines.
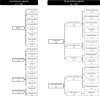
Results: Overall, 1974 publications reported 2421 studies of signals; 1683/2421 were clinical assessments of anecdotal reports of ADRs, but only 225 (13%) of these included explicit judgments on which features of the ADR reports were supportive of a signal. These 225 studies yielded 228 signals; these were supported by features, which were: 'experimental evidence' (i.e., positive dechallenge or rechallenge, 154 instances [68%]), 'temporality' (i.e., time to onset, 130 [57%]), 'exclusion of competing causes' (49 [21%]), and others (40 [17%]). Positive dechallenge/rechallenge often co-occurred with temporality (77/228). OCEBM 4 (i.e., case series and case-control studies) was the most frequent level of evidence (2078 studies). Between 2013 and 2019, there was a three-fold increase in clinical assessments of reports of ADRs compared with a less than two-fold increase in studies supported by higher levels of evidence (i.e., OCEBM 1-3). We identified an increased rate between 2013 and 2019 in disproportionality analyses (about 15 studies per year), mostly from academia.
Conclusions: Most signals were supported by temporality and dechallenge/rechallenge, but clear reporting of judgments on causality remains infrequent. The number of studies supported only by anecdotal reports of ADRs increased from year to year. The impact of a growing number of signals of disproportionate reporting communicated without an accompanying clinical assessment should be evaluated.
© 2022. The Author(s).
Conflict of interest statement
JKA has written papers on adverse drug reactions in peer-reviewed journals and has received royalties from textbooks that he has edited or co-edited; he has often acted as an expert witness in cases involving adverse drug reactions, including opioids, most often in Coroners’ courts. DS and GNN are employed by the UMC, which manages VigiBase and holds the archives of the Signal Document used to retrieve part of the included studies. Part of the reviewed materials was published in peer-reviewed journals, drug bulletins, and abstracts or posters by researchers presently or formerly belonging to this foundation. Specifically, DS has authored signals published on drug bulletins, as abstracts or posters, some of which were included in this review, and received funding for doctoral studies from the UMC. IJO has no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

