Arachnoid granulations are lymphatic conduits that communicate with bone marrow and dura-arachnoid stroma
- PMID: 36469302
- PMCID: PMC9728136
- DOI: 10.1084/jem.20220618
Arachnoid granulations are lymphatic conduits that communicate with bone marrow and dura-arachnoid stroma
Abstract
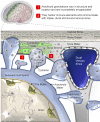
Arachnoid granulations (AG) are poorly investigated. Historical reports suggest that they regulate brain volume by passively transporting cerebrospinal fluid (CSF) into dural venous sinuses. Here, we studied the microstructure of cerebral AG in humans with the aim of understanding their roles in physiology. We discovered marked variations in AG size, lobation, location, content, and degree of surface encapsulation. High-resolution microscopy shows that AG consist of outer capsule and inner stromal core regions. The fine and porous framework suggests uncharacterized functions of AG in mechanical CSF filtration. Moreover, internal cytokine and immune cell enrichment imply unexplored neuroimmune properties of these structures that localize to the brain-meningeal lymphatic interface. Dramatic age-associated changes in AG structure are additionally identified. This study depicts for the first time microscopic networks of internal channels that communicate with perisinus spaces, suggesting that AG subserve important functions as transarachnoidal flow passageways. These data raise new theories regarding glymphatic-lymphatic coupling and mechanisms of CSF antigen clearance, homeostasis, and diseases.
© 2022 Shah et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures








Comment in
-
CSF-to-dura gateways.J Exp Med. 2023 Feb 6;220(2):e20221719. doi: 10.1084/jem.20221719. Epub 2022 Dec 6. J Exp Med. 2023. PMID: 36472584 Free PMC article.
References
-
- Absinta, M., Ha S.K., Nair G., Sati P., Luciano N.J., Palisoc M., Louveau A., Zaghloul K.A., Pittaluga S., Kipnis J., and Reich D.S.. 2017. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife. 6:e29738. 10.7554/eLife.29738 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

