Therapeutic Effects of Butyrate on Pediatric Obesity: A Randomized Clinical Trial
- PMID: 36469320
- PMCID: PMC9855301
- DOI: 10.1001/jamanetworkopen.2022.44912
Therapeutic Effects of Butyrate on Pediatric Obesity: A Randomized Clinical Trial
Erratum in
-
Error in Funding/Support.JAMA Netw Open. 2024 Jun 3;7(6):e2421429. doi: 10.1001/jamanetworkopen.2024.21429. JAMA Netw Open. 2024. PMID: 38842813 Free PMC article. No abstract available.
-
Error in Funding/Support.JAMA Netw Open. 2025 Apr 1;8(4):e259711. doi: 10.1001/jamanetworkopen.2025.9711. JAMA Netw Open. 2025. PMID: 40198076 Free PMC article. No abstract available.
Abstract
Importance: The pediatric obesity disease burden imposes the necessity of new effective strategies.
Objective: To determine whether oral butyrate supplementation as an adjunct to standard care is effective in the treatment of pediatric obesity.
Design, setting, and participants: A randomized, quadruple-blind, placebo-controlled trial was performed from November 1, 2020, to December 31, 2021, at the Tertiary Center for Pediatric Nutrition, Department of Translational Medical Science, University of Naples Federico II, Naples, Italy. Participants included children aged 5 to 17 years with body mass index (BMI) greater than the 95th percentile.
Interventions: Standard care for pediatric obesity supplemented with oral sodium butyrate, 20 mg/kg body weight per day, or placebo for 6 months was administered.
Main outcomes and measures: The main outcome was the decrease of at least 0.25 BMI SD scores at 6 months. The secondary outcomes were changes in waist circumference; fasting glucose, insulin, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglyceride, ghrelin, microRNA-221, and interleukin-6 levels; homeostatic model assessment of insulin resistance (HOMA-IR); dietary and lifestyle habits; and gut microbiome structure. Intention-to-treat analysis was conducted.
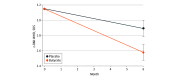
Results: Fifty-four children with obesity (31 girls [57%], mean [SD] age, 11 [2.91] years) were randomized into the butyrate and placebo groups; 4 were lost to follow-up after receiving the intervention in the butyrate group and 2 in the placebo group. At intention-to-treat analysis (n = 54), children treated with butyrate had a higher rate of BMI decrease greater than or equal to 0.25 SD scores at 6 months (96% vs 56%, absolute benefit increase, 40%; 95% CI, 21% to 61%; P < .01). At per-protocol analysis (n = 48), the butyrate group showed the following changes as compared with the placebo group: waist circumference, -5.07 cm (95% CI, -7.68 to -2.46 cm; P < .001); insulin level, -5.41 μU/mL (95% CI, -10.49 to -0.34 μU/mL; P = .03); HOMA-IR, -1.14 (95% CI, -2.13 to -0.15; P = .02); ghrelin level, -47.89 μg/mL (95% CI, -91.80 to -3.98 μg/mL; P < .001); microRNA221 relative expression, -2.17 (95% CI, -3.35 to -0.99; P < .001); and IL-6 level, -4.81 pg/mL (95% CI, -7.74 to -1.88 pg/mL; P < .001). Similar patterns of adherence to standard care were observed in the 2 groups. Baseline gut microbiome signatures predictable of the therapeutic response were identified. Adverse effects included transient mild nausea and headache reported by 2 patients during the first month of butyrate intervention.
Conclusions and relevance: Oral butyrate supplementation may be effective in the treatment of pediatric obesity.
Trial registration: ClinicalTrials.gov Identifier: NCT04620057.
Conflict of interest statement
Figures
References
-
- World Health Organization. Obesity and overweight . Accessed November 27, 2020. https://www.who.int/mediacentre/factsheets/fs311/en/

