A bacterium from a mountain lake harvests light using both proton-pumping xanthorhodopsins and bacteriochlorophyll-based photosystems
- PMID: 36469764
- PMCID: PMC9897461
- DOI: 10.1073/pnas.2211018119
A bacterium from a mountain lake harvests light using both proton-pumping xanthorhodopsins and bacteriochlorophyll-based photosystems
Abstract
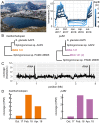
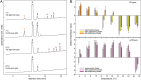
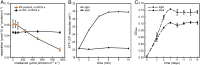
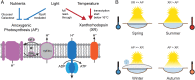
Photoheterotrophic bacteria harvest light energy using either proton-pumping rhodopsins or bacteriochlorophyll (BChl)-based photosystems. The bacterium Sphingomonas glacialis AAP5 isolated from the alpine lake Gossenköllesee contains genes for both systems. Here, we show that BChl is expressed between 4°C and 22°C in the dark, whereas xanthorhodopsin is expressed only at temperatures below 16°C and in the presence of light. Thus, cells grown at low temperatures under a natural light-dark cycle contain both BChl-based photosystems and xanthorhodopsins with a nostoxanthin antenna. Flash photolysis measurements proved that both systems are photochemically active. The captured light energy is used for ATP synthesis and stimulates growth. Thus, S. glacialis AAP5 represents a chlorophototrophic and a retinalophototrophic organism. Our analyses suggest that simple xanthorhodopsin may be preferred by the cells under higher light and low temperatures, whereas larger BChl-based photosystems may perform better at lower light intensities. This indicates that the use of two systems for light harvesting may represent an evolutionary adaptation to the specific environmental conditions found in alpine lakes and other analogous ecosystems, allowing bacteria to alternate their light-harvesting machinery in response to large seasonal changes of irradiance and temperature.
Keywords: anoxygenic photosynthesis; bacteriochlorophyll a; dual phototrophy; light energy; xanthorhodopsin.
Conflict of interest statement
The authors declare no competing interest.
Figures


Comment in
-
Bacterial dual phototrophy was demystified.Trends Microbiol. 2023 Apr;31(4):326-328. doi: 10.1016/j.tim.2023.02.005. Epub 2023 Feb 21. Trends Microbiol. 2023. PMID: 36822951
References
-
- Yurkov V. V., Csotonyi J. T., “New light on aerobic anoxygenic phototrophs” in The Purple Phototrophic Bacteria, Hunter C. N., Daldal F., , M. C. Thurnauer, Beatty J. T., Eds. (Advances in Photosynthesis and Respiration, Springer, 2009), vol. 28, pp. 31–55.
-
- Koblížek M., Ecology of aerobic anoxygenic phototrophs in aquatic environments. FEMS Microbiol. Rev. 39, 854–870 (2015). - PubMed
-
- Kolber Z. S., et al. , Contribution of aerobic photoheterotrophic bacteria to the carbon cycle in the ocean. Science 292, 2492–2495 (2001). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

