An abstract categorical decision code in dorsal premotor cortex
- PMID: 36469775
- PMCID: PMC9897443
- DOI: 10.1073/pnas.2214562119
An abstract categorical decision code in dorsal premotor cortex
Erratum in
-
Correction for Diaz-deLeon et al., An abstract categorical decision code in dorsal premotor cortex.Proc Natl Acad Sci U S A. 2023 Mar 14;120(11):e2302615120. doi: 10.1073/pnas.2302615120. Epub 2023 Mar 9. Proc Natl Acad Sci U S A. 2023. PMID: 36893283 Free PMC article. No abstract available.
Abstract
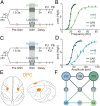
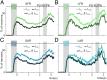
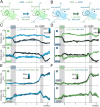
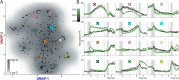
The dorsal premotor cortex (DPC) has classically been associated with a role in preparing and executing the physical motor variables during cognitive tasks. While recent work has provided nuanced insights into this role, here we propose that DPC also participates more actively in decision-making. We recorded neuronal activity in DPC while two trained monkeys performed a vibrotactile categorization task, utilizing two partially overlapping ranges of stimulus values that varied on two physical attributes: vibrotactile frequency and amplitude. We observed a broad heterogeneity across DPC neurons, the majority of which maintained the same response patterns across attributes and ranges, coding in the same periods, mixing temporal and categorical dynamics. The predominant categorical signal was maintained throughout the delay, movement periods and notably during the intertrial period. Putting the entire population's data through two dimensionality reduction techniques, we found strong temporal and categorical representations without remnants of the stimuli's physical parameters. Furthermore, projecting the activity of one population over the population axes of the other yielded identical categorical and temporal responses. Finally, we sought to identify functional subpopulations based on the combined activity of all stimuli, neurons, and time points; however, we found that single-unit responses mixed temporal and categorical dynamics and couldn't be clustered. All these point to DPC playing a more decision-related role than previously anticipated.
Keywords: categorical decision; continuum responses; dorsal premotor cortex; inter-trial coding; temporal signals.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

