Prolonged smoldering Douglas fir smoke inhalation augments respiratory resistances, stiffens the aorta, and curbs ejection fraction in hypercholesterolemic mice
- PMID: 36470384
- PMCID: PMC10699119
- DOI: 10.1016/j.scitotenv.2022.160609
Prolonged smoldering Douglas fir smoke inhalation augments respiratory resistances, stiffens the aorta, and curbs ejection fraction in hypercholesterolemic mice
Abstract
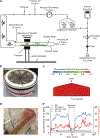
While mounting evidence suggests that wildland fire smoke (WFS) inhalation may increase the burden of cardiopulmonary disease, the occupational risk of repeated exposure during wildland firefighting remains unknown. To address this concern, we evaluated the cardiopulmonary function in mice following a cumulative exposure to lab-scale WFS equivalent to a mid-length wildland firefighter (WLFF) career. Dosimetry analysis indicated that 80 exposure hours at a particulate concentration of 22 mg/m3 yield in mice the same cumulative deposited mass per unit of lung surface area as 3600 h of wildland firefighting. To satisfy this condition, male Apoe-/- mice were whole-body exposed to either air or smoldering Douglas fir smoke (DFS) for 2 h/day, 5 days/week, over 8 consecutive weeks. Particulate size in DFS fell within the respirable range for both mice and humans, with a count median diameter of 110 ± 20 nm. Expiratory breath hold in mice exposed to DFS significantly reduced their minute volume (DFS: 27 ± 4; Air: 122 ± 8 mL/min). By the end of the exposure time frame, mice in the DFS group exhibited a thicker (DFS: 109 ± 3; Air: 98 ± 3 μm) and less distensible (DFS: 23 ± 1; Air: 28 ± 1 MPa-1) aorta with reduced diastolic blood augmentation capacity (DFS: 53 ± 2; Air: 63 ± 2 kPa). Cardiac magnetic resonance imaging further revealed larger end-systolic volume (DFS: 14.6 ± 1.1; Air: 9.9 ± 0.9 μL) and reduced ejection-fraction (DFS: 64.7 ± 1.0; Air: 75.3 ± 0.9 %) in mice exposed to DFS. Consistent with increased airway epithelium thickness (DFS: 10.4 ± 0.8; Air: 7.6 ± 0.3 μm), airway Newtonian resistance was larger following DFS exposure (DFS: 0.23 ± 0.03; Air: 0.20 ± 0.03 cmH2O-s/mL). Furthermore, parenchyma mean linear intercept (DFS: 36.3 ± 0.8; Air: 33.3 ± 0.8 μm) and tissue thickness (DFS: 10.1 ± 0.5; Air: 7.4 ± 0.7 μm) were larger in DFS mice. Collectively, mice exposed to DFS manifested early signs of cardiopulmonary dysfunction aligned with self-reported events in mid-career WLFFs.
Keywords: Airway morphometry; Aortic distensibility; Carboxyhemoglobin; Dosimetry; Particulate matter; Wildland fire smoke.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Caton SE, Hakes RS, Gorham DJ, Zhou A, Gollner MJ, Review of pathways for building fire spread in the wildland urban interface part I: exposure conditions, Fire technology 53 (2) (2017) 429–473. doi:10.1007/s10694-016-0601-7. - DOI
-
- Intergovernmental Panel On Climate Change, Climate change: Impact, adaptation, vulnerability, Available at https://www.ipcc.ch/report/ar5/wg2/ [Online; accessed 12-Nov-2021] (2014).
-
- Rice MB, Henderson SB, Lambert AA, Cromar KR, Hall JA, Cascio WE, Smith PG, Marsh BJ, Coefield S, Balmes JR, et al., Respiratory impacts of wildland fire smoke: Future challenges and policy opportunities. an official american thoracic society workshop report, Annals of the American Thoracic Society 18 (6) (2021) 921–930. doi:10.1513/AnnalsATS.202102-148ST. - DOI - PMC - PubMed
-
- US Environmental Protection Agency, Air emissions inventories. criteria pollutants national tier 1 for 1970 – 2020. Available at https://www.epa.gov/air-emissions-inventories [Online; accessed 12-Nov-2021].
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

