The TGM2 inhibitor cysteamine hydrochloride does not impact corneal epithelial and stromal wound healing in vitro and in vivo
- PMID: 36470430
- PMCID: PMC10120528
- DOI: 10.1016/j.exer.2022.109338
The TGM2 inhibitor cysteamine hydrochloride does not impact corneal epithelial and stromal wound healing in vitro and in vivo
Abstract
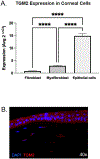
Corneal wound healing is integral for resolution of corneal disease or for post-operative healing. However, corneal scarring that may occur secondary to this process can significantly impair vision. Tissue transglutaminase 2 (TGM2) inhibition has shown promising antifibrotic effects and thus holds promise to prevent or treat corneal scarring. The commercially available ocular solution for treatment of ocular manifestations of Cystinosis, Cystaran®, contains the TGM2 inhibitor cysteamine hydrochloride (CH). The purpose of this study is to assess the safety of CH on corneal epithelial and stromal wounds, its effects on corneal wound healing, and its efficacy against corneal scarring following wounding. Quantitative polymerase chain reaction (qPCR) and immunohistochemistry (IHC) were first used to quantify and localize TGM2 expression in the cornea. Subsequently, (i) the in vitro effects of CH at 0.163, 1.63, and 16.3 mM on corneal epithelial cell migration was assessed with an epithelial cell migration assay, and (ii) the in vivo effects of application of 1.63 mM CH on epithelial and stromal wounds was assessed in a rabbit model with ophthalmic examinations, inflammation scoring, color and fluorescein imaging, optical coherence tomography (OCT), and confocal biomicroscopy. Post-mortem assessment of corneal tissue post-stromal wounding included biomechanical characterization (atomic force microscopy (AFM)), histology (H&E staining), and determining incidence of myofibroblasts (immunostaining against α-SMA) in wounded corneal tissue. TGM2 expression was highest in corneal epithelial cells. Application of the TGM2 inhibitor CH did not affect in vitro epithelial cell migration at the two lower concentrations tested. At 16.3 mM, decreased cell migration was observed. In vivo application of CH at 57 mM was well tolerated and did not adversely affect wound healing. No difference in corneal scarring was found between CH treated and vehicle control eyes. This study shows that the TGM2 inhibitor CH, at the FDA-approved dose, is well tolerated in a rabbit model of corneal wound healing and does not adversely affect epithelial or stromal wound healing. This supports the safe use of this medication in Cystinosis patients with open corneal wounds. CH did not have an effect on corneal scarring in this study, suggesting that Cystaran® administration to patients with corneal wounds is unlikely to decrease corneal fibrosis.
Keywords: Corneal wound healing; Cystaran; Cysteamine hydrochloride; Fibrosis; Phototherapeutic keratectomy; Scarring; Tissue transglutaminase 2.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors have no conflicts to disclose.
Figures



References
-
- Aragona P, Aguennouz M, Rania L, Postorino E, Sommario MS, Roszkowska AM, et al., 2015. Matrix metalloproteinase 9 and transglutaminase 2 expression at the ocular surface in patients with different forms of dry eye disease. Ophthalmology 122 (1), 62–71. - PubMed
-
- Boneham GC, Collin HB, 1995. Steroid inhibition of limbal blood and lymphatic vascular cell growth. Curr. Eye Res 14 (1), 1–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

