Olfactory dysfunction in COVID-19: new insights into the underlying mechanisms
- PMID: 36470705
- PMCID: PMC9666374
- DOI: 10.1016/j.tins.2022.11.003
Olfactory dysfunction in COVID-19: new insights into the underlying mechanisms
Abstract
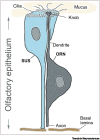
The mechanisms of olfactory dysfunction in COVID-19 are still unclear. In this review, we examine potential mechanisms that may explain why the sense of smell is lost or altered. Among the current hypotheses, the most plausible is that death of infected support cells in the olfactory epithelium causes, besides altered composition of the mucus, retraction of the cilia on olfactory receptor neurons, possibly because of the lack of support cell-derived glucose in the mucus, which powers olfactory signal transduction within the cilia. This mechanism is consistent with the rapid loss of smell with COVID-19, and its rapid recovery after the regeneration of support cells. Host immune responses that cause downregulation of genes involved in olfactory signal transduction occur too late to trigger anosmia, but may contribute to the duration of the olfactory dysfunction.
Keywords: SARS-CoV-2; anosmia; olfactory epithelium; parosmia; smell loss; sustentacular cell.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
-
- Dhurvey V., et al. Two worst pandemics- Spanish Flu and COVID-19: a review. Magna Sci. Adv. Biol. Pharm. 2021;4:1–12.
-
- Edwards S.N. Understanding the present through the past: a comparison of Spanish news coverage of the 1918 flu and COVID-19 pandemics. J. Mass. Commun. Q. 2022;99:12–43.
-
- Leyden E., Guttmann S., editors. Auftrage des Vereins für Innere Medicin in Berlin. Verlag J.F. Bergmann; 1892.
-
- Hwang C.S. Olfactory neuropathy in severe acute respiratory syndrome: report of a case. Acta Neurol. Taiwanica. 2006;15:26–28. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

