A library of base editors for the precise ablation of all protein-coding genes in the mouse mitochondrial genome
- PMID: 36470976
- PMCID: PMC10195678
- DOI: 10.1038/s41551-022-00968-1
A library of base editors for the precise ablation of all protein-coding genes in the mouse mitochondrial genome
Abstract
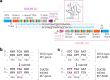
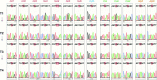
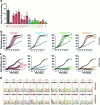
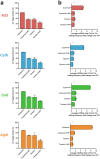
The development of curative treatments for mitochondrial diseases, which are often caused by mutations in mitochondrial DNA (mtDNA) that impair energy metabolism and other aspects of cellular homoeostasis, is hindered by an incomplete understanding of the underlying biology and a scarcity of cellular and animal models. Here we report the design and application of a library of double-stranded-DNA deaminase-derived cytosine base editors optimized for the precise ablation of every mtDNA protein-coding gene in the mouse mitochondrial genome. We used the library, which we named MitoKO, to produce near-homoplasmic knockout cells in vitro and to generate a mouse knockout with high heteroplasmy levels and no off-target edits. MitoKO should facilitate systematic and comprehensive investigations of mtDNA-related pathways and their impact on organismal homoeostasis, and aid the generation of clinically meaningful in vivo models of mtDNA dysfunction.
© 2022. The Author(s).
Conflict of interest statement
M.M. is a founder, shareholder and member of the Scientific Advisory Board of Pretzel Therapeutics, Inc. P.S-P., P.A.N., L.V.H., and C.D.M. provide consultancy services for Pretzel Therapeutics. P.S-P., C.D.M and M.M. have filed patent applications on this work. L.V.H. is director of NextGenSeek Ltd. The remaining authors declare no competing interests.
Figures








Comment in
-
Ablating all mitochondrial protein-coding genes.Nat Biomed Eng. 2023 May;7(5):609-611. doi: 10.1038/s41551-022-00993-0. Nat Biomed Eng. 2023. PMID: 36564630 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

