Efficacy of empagliflozin in heart failure with preserved versus mid-range ejection fraction: a pre-specified analysis of EMPEROR-Preserved
- PMID: 36471037
- PMCID: PMC9800272
- DOI: 10.1038/s41591-022-02041-5
Efficacy of empagliflozin in heart failure with preserved versus mid-range ejection fraction: a pre-specified analysis of EMPEROR-Preserved
Abstract
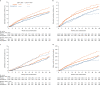
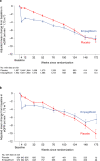
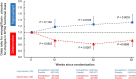
The EMPEROR-Preserved trial showed that the sodium-glucose co-transporter 2 inhibitor empagliflozin significantly reduces the risk of cardiovascular death or hospitalization for heart failure (HHF) in heart failure patients with left ventricular ejection fraction (LVEF) > 40%. Here, we report the results of a pre-specified analysis that separately evaluates these patients stratified by LVEF: preserved (≥ 50%) (n = 4,005; 66.9%) or mid-range (41-49%). In patients with LVEF ≥ 50%, empagliflozin reduced the risk of cardiovascular death or HHF (the primary endpoint) by 17% versus placebo (hazard ratio (HR) 0.83; 95% confidence interval (CI): 0.71-0.98, P = 0.024). For the key secondary endpoint, the HR for total HHF was 0.83 (95%CI: 0.66-1.04, P = 0.11). For patients with an LVEF of 41-49%, the HR for empagliflozin versus placebo was 0.71 (95%CI: 0.57-0.88, P = 0.002) for the primary outcome (Pinteraction = 0.27), and 0.57 (95%CI: 0.42-0.79, P < 0.001) for total HHF (Pinteraction = 0.06). These results, together with those from the EMPEROR-Reduced trial in patients with LVEF < 40%, support the use of empagliflozin across the full spectrum of LVEF in heart failure.
© 2022. The Author(s).
Conflict of interest statement
Figures



References
-
- Ponikowski P, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. - DOI - PubMed
-
- Bozkurt B, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J. Card. Fail. 2021;27:387–413. doi: 10.1016/j.cardfail.2021.01.022. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

