Biopharmaceutical benchmarks 2022
- PMID: 36471135
- PMCID: PMC9735008
- DOI: 10.1038/s41587-022-01582-x
Biopharmaceutical benchmarks 2022
Abstract
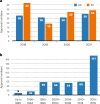
Monoclonal antibodies as a group continue to lead biopharmaceuticals in numbers of approvals and sales, although COVID-19 vaccines shot to the top of the list of highest-grossing individual products.
Figures


References
-
- Kelley, B., Kiss, R. & Laird, M. (2018). in New Bioprocessing Strategies: Development and Manufacturing of Recombinant Antibodies and Proteins (eds Kiss, B., Gottschalk, U. & Pohlscheidt, M.) 443–462 (Springer, 2018). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

