Neoadjuvant chemotherapy for patients with international federation of gynecology and obstetrics stages IB3 and IIA2 cervical cancer: a multicenter prospective trial
- PMID: 36471257
- PMCID: PMC9724322
- DOI: 10.1186/s12885-022-10355-3
Neoadjuvant chemotherapy for patients with international federation of gynecology and obstetrics stages IB3 and IIA2 cervical cancer: a multicenter prospective trial
Abstract
Background: Preoperative neoadjuvant chemotherapy (NACT) has been widely used in developing countries for the treatment of patients with International Federation of Gynecology and Obstetrics (FIGO) stages IB3 and IIA2 cervical cancer. However, the effectiveness of NACT and treatment options for NACT-insensitive patients have been concerning. This study will assess prognostic differences between NACT and primary surgery treatment (PST), determine factors associated with prognosis, and explore better adjuvant treatment modalities for NACT-insensitive patients.
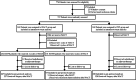
Methods: This study analyzed clinical characteristics, pathological characteristics, treatment options, and follow-up information of 774 patients with FIGO stages IB3 and IIA2 cervical cancer from 28 centers from January 2016 to October 2019 who participated in a multicenter, prospective, randomized controlled trial.
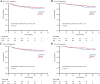
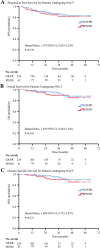
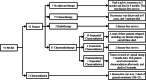
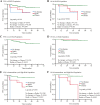
Results: For patients undergoing NACT, the 5-year OS and PFS rate was 85.8 and 80.5% respectively. They were similar in the PST group. There was no significant difference in OS and PFS between clinical response (CR)/partial response (PR) groups and stable disease (SD)/progressive disease (PD) groups. Apart from deep cervical invasion (p = 0.046) affecting OS for patients undergoing NACT, no other clinical and pathological factors were associated with OS. 97.8% of NACT-insensitive patients opted for surgery. If these patients did not have intermediate- or high-risk factors, whether they had undergone postoperative adjuvant therapy was irrelevant to their prognosis, whereas for patients with intermediate- or high-risk factors, adjuvant chemotherapy resulted in better PFS (chemotherapy vs. no therapy, p < 0.001; chemotherapy vs. radiotherapy, p = 0.019) and OS (chemotherapy vs. no therapy, p < 0.001; chemotherapy vs. radiotherapy, p = 0.002).
Conclusions: NACT could be a choice for patients with FIGO stages IB3 and IIA2 cervical cancer. The main risk factor influencing prognosis in the NACT group is deep cervical invasion. After systematic treatment, insensitivity to NACT does not indicate a poorer prognosis. For NACT-insensitive patients, Chinese prefer surgery. Postoperative adjuvant therapy in patients with no intermediate- or high-risk factors does not improve prognosis, and chemotherapy in patients with intermediate- and high-risk factors is more effective than radiation therapy and other treatments.
Trial registration: The study was prospectively registered on ClinicalTrials.gov (NCT03308591); date of registration: 12/10/2017.
Keywords: Cervical cancer; Neoadjuvant chemotherapy; Non-responders; Prognosis; Radical surgery.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Colombo N, Carinelli S, Colombo A, Marini C, Rollo D, Sessa C. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23 Suppl 7:vii27–32. - PubMed
-
- (NCCN). NCCN. NCCN Clinical Practice Guidelines in Oncology. Cervical Cancer. Version 1 .2022 - October 26, 2021 [Available from: https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf.

