Personalised rehabilitation to improve return to work in patients with persistent spinal pain syndrome type II after spinal cord stimulation implantation: a study protocol for a 12-month randomised controlled trial-the OPERA study
- PMID: 36471349
- PMCID: PMC9721015
- DOI: 10.1186/s13063-022-06895-5
Personalised rehabilitation to improve return to work in patients with persistent spinal pain syndrome type II after spinal cord stimulation implantation: a study protocol for a 12-month randomised controlled trial-the OPERA study
Abstract
Background: For patients with therapy-refractory persistent spinal pain syndrome type II (PSPS-T2), spinal cord stimulation (SCS) may serve as an effective minimally invasive treatment. Despite the evidence that SCS can improve return to work (RTW), only 9.5 to 14% of patients implanted with SCS are effectively capable of returning to work. Thus, it seems that current post-operative interventions are not effective for achieving RTW after SCS implantation in clinical practice. The current objective is to examine whether a personalised biopsychosocial rehabilitation programme specifically targeting RTW alters the work ability in PSPS-T2 patients after SCS implantation compared to usual care.
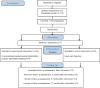
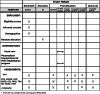
Methods: A two-arm, parallel-group multicentre randomised controlled trial will be conducted including 112 patients who will be randomised (1:1) to either (a) a personalised biopsychosocial RTW rehabilitation programme of 14 weeks or (b) a usual care arm, both with a follow-up period until 12 months after the intervention. The primary outcome is work ability. The secondary outcomes are work status and participation, pain intensity, health-related quality of life, physical activity and functional disability, functional capacities, sleep quality, kinesiophobia, self-management, anxiety, depression and healthcare expenditure.
Discussion: Within the OPERA project, we propose a multidisciplinary personalised biopsychosocial rehabilitation programme specifically targeting RTW for patients implanted with SCS, to tackle the high socio-economic burden of patients that are not re-entering the labour market. The awareness is growing that the burden of PSPS-T2 on our society is expected to increase over time due to the annual increase of spinal surgeries. However, innovative and methodologically rigorous trials exploring the potential to decrease the socio-economic burden when patients initiate a trajectory with SCS are essentially lacking.
Trial registration: ClinicalTrials.gov NCT05269212. Registered on 7 March 2022.
Keywords: Failed back surgery syndrome; Neuromodulation; Personalised medicine; Randomised controlled trial; Rehabilitation.
© 2022. The Author(s).
Conflict of interest statement
Lisa Goudman is a postdoctoral research fellow funded by the Research Foundation Flanders (FWO), Belgium (project number 12ZF622N). Maarten Moens has received speaker fees from Medtronic, Nevro and Saluda Medical. STIMULUS received independent research grants from Medtronic. There are no other conflicts of interest to declare.
Figures
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

