Proteomic profiling of circulating plasma exosomes reveals novel biomarkers of Alzheimer's disease
- PMID: 36471423
- PMCID: PMC9720984
- DOI: 10.1186/s13195-022-01133-1
Proteomic profiling of circulating plasma exosomes reveals novel biomarkers of Alzheimer's disease
Abstract
Background: Neuronal- and astrocyte-derived exosomes have been identified as an optimal source for screening biomarkers for Alzheimer's disease (AD). However, few studies focus on the bulk exosome population isolated from plasma of AD. This study investigated whether proteins in bulk exosomes can aid in the diagnosis of AD.
Methods: The plasma exosomes were collected by ultracentrifuge. Protein samples were extracted from exosomes. Cerebrospinal fluid levels of amyloid β (Aβ)42 and phosphorylated tau (P-tau)181 were measured for diagnostic purposes. A pilot study (controls, 20; AD, 20) followed by a second dataset (controls, 56; AD, 58) was used to establish a diagnostic model of AD. Mass spectrometry-based proteomics was performed to profile the plasma exosomal proteome. Parallel reaction monitoring was used to further confirm the differentially expressed proteins.
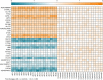
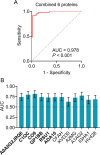
Results: In total, 328 proteins in plasma exosomes were quantified. Among them, 31 proteins were altered in AD patients, and 12 were validated. The receiver operating characteristic curve analysis revealed a combination of six proteins (upregulated: Ig-like domain-containing protein (A0A0G2JRQ6), complement C1q subcomponent subunit C (C1QC), complement component C9 (CO9), platelet glycoprotein Ib beta chain (GP1BB), Ras suppressor protein 1 (RSU1); downregulated: disintegrin and metalloproteinase domain 10 (ADA10)) has the capacity to differentiate AD patients from healthy controls with high accuracy. Linear correlation analysis showed that the combination was significantly correlated with cognitive performance.
Conclusions: The combination of plasma exosomal proteins A0A0G2JRQ6, C1QC, CO9, GP1BB, RSU1, and ADA10 acts as a novel candidate biomarker to differentiate AD patients from healthy individuals.
Keywords: Alzheimer’s disease; Biomarker; Diagnosis; Exosome; Proteomics.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

