Superficial Tale of Two Functional Groups: On the Surface Propensity of Aqueous Carboxylic Acids, Alkyl Amines, and Amino Acids
- PMID: 36472092
- PMCID: PMC9730837
- DOI: 10.1021/acs.accounts.2c00494
Superficial Tale of Two Functional Groups: On the Surface Propensity of Aqueous Carboxylic Acids, Alkyl Amines, and Amino Acids
Abstract
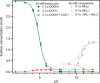
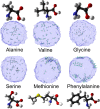
The gas-liquid interface of water is environmentally relevant due to the abundance of aqueous aerosol particles in the atmosphere. Aqueous aerosols often contain a significant fraction of organics. As aerosol particles are small, surface effects are substantial but not yet well understood. One starting point for studying the surface of aerosols is to investigate the surface of aqueous solutions. We review here studies of the surface composition of aqueous solutions using liquid-jet photoelectron spectroscopy in combination with theoretical simulations. Our focus is on model systems containing two functional groups, the carboxylic group and the amine group, which are both common in atmospheric organics. For alkanoic carboxylic acids and alkyl amines, we find that the surface propensity of such amphiphiles can be considered to be a balance between the hydrophilic interactions of the functional group and the hydrophobic interactions of the alkyl chain. For the same chain length, the neutral alkyl amine has a lower surface propensity than the neutral alkanoic carboxylic acid, whereas the surface propensity of the corresponding alkyl ammonium ion is higher than that of the alkanoic carboxylate ion. This different propensity leads to a pH-dependent surface composition which differs from the bulk, with the neutral forms having a much higher surface propensity than the charged ones. In aerosols, alkanoic carboxylic acids and alkyl amines are often found together. For such mixed systems, we find that the oppositely charged molecular ions form ion pairs at the surface. This cooperative behavior leads to a more organic-rich and hydrophobic surface than would be expected in a wide, environmentally relevant pH range. Amino acids contain a carboxylic and an amine group, and amino acids of biological origin are found in aerosols. Depending on the side group, we observe surface propensity ranging from surface-depleted to enriched by a factor of 10. Cysteine contains one more titratable group, which makes it exhibit more complex behavior, with some protonation states found only at the surface and not in the bulk. Moreover, the presence of molecular ions at the surface is seen to affect the distribution of inorganic ions. As the charge of the molecular ions changes with protonation, the effects on the inorganic ions also exhibit a pH dependence. Our results show that for these systems the surface composition differs from the bulk and changes with pH and that the results obtained for single-component solutions may be modified by ion-ion interactions in the case of mixed solutions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Surfaces of Atmospheric Droplet Models Probed with Synchrotron XPS on a Liquid Microjet.Acc Chem Res. 2024 Jan 16;57(2):177-187. doi: 10.1021/acs.accounts.3c00201. Epub 2023 Dec 29. Acc Chem Res. 2024. PMID: 38156821 Free PMC article.
-
Cooperative and competitive effects in pH-dependent surface composition of atmospherically relevant organic ions in water.Phys Chem Chem Phys. 2025 Mar 12;27(11):5791-5797. doi: 10.1039/d4cp04287e. Phys Chem Chem Phys. 2025. PMID: 40019161
-
Chemical equilibria of aqueous ammonium-carboxylate systems in aqueous bulk, close to and at the water-air interface.Phys Chem Chem Phys. 2019 Jun 21;21(23):12434-12445. doi: 10.1039/c9cp02449b. Epub 2019 May 30. Phys Chem Chem Phys. 2019. PMID: 31143906
-
Coupling of electron transfer to proton uptake at the Q(B) site of the bacterial reaction center: a perspective from FTIR difference spectroscopy.Biochim Biophys Acta. 2008 Oct;1777(10):1229-48. doi: 10.1016/j.bbabio.2008.06.012. Epub 2008 Jul 11. Biochim Biophys Acta. 2008. PMID: 18671937 Review.
-
Polymers in the Medical Antiviral Front-Line.Polymers (Basel). 2020 Jul 31;12(8):1727. doi: 10.3390/polym12081727. Polymers (Basel). 2020. PMID: 32752109 Free PMC article. Review.
Cited by
-
Photoelectron circular dichroism of aqueous-phase alanine.Chem Sci. 2025 Apr 7;16(20):8637-8647. doi: 10.1039/d5sc00167f. eCollection 2025 May 21. Chem Sci. 2025. PMID: 40255964 Free PMC article.
-
Interaction of ions and surfactants at the seawater-air interface.Environ Sci Atmos. 2025 Feb 3;5(3):291-299. doi: 10.1039/d4ea00151f. eCollection 2025 Mar 13. Environ Sci Atmos. 2025. PMID: 39989667 Free PMC article.
-
From Gas to Solution: The Changing Neutral Structure of Proline upon Solvation.J Phys Chem A. 2024 Nov 28;128(47):10202-10212. doi: 10.1021/acs.jpca.4c05628. Epub 2024 Nov 13. J Phys Chem A. 2024. PMID: 39536145 Free PMC article.
References
-
- Ekholm V.; Caleman C.; Bjärnhall Prytz N.; Walz M.-M.; Werner J.; Öhrwall G.; Rubensson J.-E.; Björneholm O. Strong Enrichment of Atmospherically Relevant Organic Ions at the Aqueous Interface: The Role of Ion Pairing and Cooperative Effects. Phys. Chem. Chem. Phys. 2018, 20, 27185.10.1039/C8CP04525A. - DOI - PubMed
-
- Climate Change 2022: Impacts, Adaptation and Vulnerability | Climate Change 2022: Impacts, Adaptation and Vulnerability. https://www.ipcc.ch/report/ar6/wg2/ (accessed July 10, 2022).
-
- Jimenez J. L.; Canagaratna M. R.; Donahue N. M.; Prevot A. S. H.; Zhang Q.; Kroll J. H.; DeCarlo P. F.; Allan J. D.; Coe H.; Ng N. L.; Aiken A. C.; Docherty K. S.; Ulbrich I. M.; Grieshop A. P.; Robinson A. L.; Duplissy J.; Smith J. D.; Wilson K. R.; Lanz V. A.; Hueglin C.; Sun Y. L.; Tian J.; Laaksonen A.; Raatikainen T.; Rautiainen J.; Vaattovaara P.; Ehn M.; Kulmala M.; Tomlinson J. M.; Collins D. R.; Cubison M. J.; Dunlea J.; Huffman J. A.; Onasch T. B.; Alfarra M. R.; Williams P. I.; Bower K.; Kondo Y.; Schneider J.; Drewnick F.; Borrmann S.; Weimer S.; Demerjian K.; Salcedo D.; Cottrell L.; Griffin R.; Takami A.; Miyoshi T.; Hatakeyama S.; Shimono A.; Sun J. Y.; Zhang Y. M.; Dzepina K.; Kimmel J. R.; Sueper D.; Jayne J. T.; Herndon S. C.; Trimborn A. M.; Williams L. R.; Wood E. C.; Middlebrook A. M.; Kolb C. E.; Baltensperger U.; Worsnop D. R. Evolution of organic aerosols in the atmosphere. Science 2009, 326, 1525.10.1126/science.1180353. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

