Effectiveness and tolerability of dual and triple combination inhaler therapies compared with each other and varying doses of inhaled corticosteroids in adolescents and adults with asthma: a systematic review and network meta-analysis
- PMID: 36472162
- PMCID: PMC9723963
- DOI: 10.1002/14651858.CD013799.pub2
Effectiveness and tolerability of dual and triple combination inhaler therapies compared with each other and varying doses of inhaled corticosteroids in adolescents and adults with asthma: a systematic review and network meta-analysis
Abstract
Background: Current guidelines recommend a higher-dose inhaled corticosteroids (ICS) or adding a long-acting muscarinic antagonist (LAMA) when asthma is not controlled with medium-dose (MD) ICS/long-acting beta2-agonist (LABA) combination therapy.
Objectives: To assess the effectiveness and safety of dual (ICS/LABA) and triple therapies (ICS/LABA/LAMA) compared with each other and with varying doses of ICS in adolescents and adults with uncontrolled asthma.
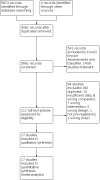
Search methods: We searched multiple databases for pre-registered randomised controlled trials (RCTs) of at least 12 weeks of study duration from 2008 to 18 February 2022.
Selection criteria: We searched studies, including adolescents and adults with uncontrolled asthma who had been treated with, or were eligible for, MD-ICS/LABA, comparing dual and triple therapies. We excluded cluster- and cross-over RCTs.
Data collection and analysis: We conducted a systematic review and network meta-analysis according to the previously published protocol. We used Cochrane's Screen4ME workflow to assess search results and Grading of Recommendations Assessment, Development and Evaluation (GRADE) to assess the certainty of evidence. The primary outcome was steroid-requiring asthma exacerbations and asthma-related hospitalisations (moderate to severe and severe exacerbations).
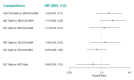
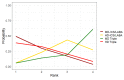
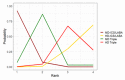
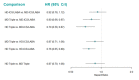
Main results: We included 17,161 patients with uncontrolled asthma from 17 studies (median duration 26 weeks; mean age 49.1 years; male 40%; white 81%; mean forced expiratory volume in 1 second (MEF 1)1.9 litres and 61% predicted). The quality of included studies was generally good except for some outcomes in a few studies due to high attrition rates. Medium-dose (MD) and high-dose (HD) triple therapies reduce steroid-requiring asthma exacerbations (hazard ratio (HR) 0.84 [95% credible interval (CrI) 0.71 to 0.99] and 0.69 [0.58 to 0.82], respectively) (high-certainty evidence), but not asthma-related hospitalisations, compared to MD-ICS/LABA. High-dose triple therapy likely reduces steroid-requiring asthma exacerbations compared to MD triple therapy (HR 0.83 [95% CrI 0.69 to 0.996], [moderate certainty]). Subgroup analyses suggest the reduction in steroid-requiring exacerbations associated with triple therapies may be only for those with a history of asthma exacerbations in the previous year but not for those without. High-dose triple therapy, but not MD triple, results in a reduction in all-cause adverse events (AEs) and likely reduces dropouts due to AEs compared to MD-ICS/LABA (odds ratio (OR) 0.79 [95% CrI 0.69 to 0.90], [high certainty] and 0.50 [95% CrI 0.30 to 0.84], [moderate certainty], respectively). Triple therapy results in little to no difference in all-cause or asthma-related serious adverse events (SAEs) compared to dual therapy (high certainty). The evidence suggests triple therapy results in little or no clinically important difference in symptoms or quality of life compared to dual therapy considering the minimal clinically important differences (MCIDs) and HD-ICS/LABA is unlikely to result in any significant benefit or harm compared to MD-ICS/LABA.
Authors' conclusions: Medium-dose and HD triple therapies reduce steroid-requiring asthma exacerbations, but not asthma-related hospitalisations, compared to MD-ICS/LABA especially in those with a history of asthma exacerbations in the previous year. High-dose triple therapy is likely superior to MD triple therapy in reducing steroid-requiring asthma exacerbations. Triple therapy is unlikely to result in clinically meaningful improvement in symptoms or quality of life compared to dual therapy considering the MCIDs. High-dose triple therapy, but not MD triple, results in a reduction in all-cause AEs and likely reduces dropouts due to AEs compared to MD-ICS/LABA. Triple therapy results in little to no difference in all-cause or asthma-related SAEs compared to dual therapy. HD-ICS/LABA is unlikely to result in any significant benefit or harm compared to MD-ICS/LABA, although long-term safety of higher rather than MD- ICS remains to be demonstrated given the median duration of included studies was six months. The above findings may assist deciding on a treatment option when asthma is not controlled with MD-ICS/LABA.
Trial registration: ClinicalTrials.gov NCT00424008 NCT01686633 NCT01099722 NCT00646594 NCT03158311 NCT00772538 NCT00776984 NCT02571777 NCT02924688 NCT02175771 NCT00394368 NCT00651768 NCT01475721 NCT02554786 NCT02676076 NCT02676089 NCT00381485 NCT01147848 NCT01202084 NCT00901368 NCT00350207 NCT02301975 NCT01018186 NCT01257230 NCT01277523 NCT03358147 NCT00379288 NCT01570478 NCT02892344 NCT03376932 NCT01244984 NCT01340209 NCT01316380 NCT00565266 NCT02139644 NCT03063086 NCT01290874 NCT01471340 NCT03387241 NCT04191434 NCT04191447 NCT04609878 NCT04609904.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Y. Oba: has provided consultation and received honoraria from Genentech unrelated to the current review.
T Patel: none known.
S Anwer: none known.
T Maduke: none known.
S Dias: none known.
Figures

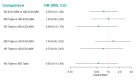
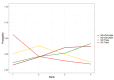

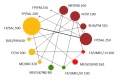
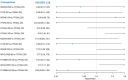










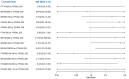
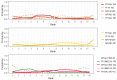

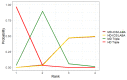

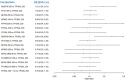




























Update of
- doi: 10.1002/14651858.CD013799
References
References to studies included in this review
Bernstein 2011 {published and unpublished data}
-
- Bernstein DI, Hébert J, Cheema A, Murphy KR, Chérrez-Ojeda I, Matiz-Bueno CE, et al. Efficacy and onset of action of mometasone furoate/formoterol and fluticasone propionate/salmeterol combination treatment in subjects with persistent asthma. Allergy Asthma and Clinical Immunology 2011;7(1):21. - PMC - PubMed
Bernstein 2015 {published and unpublished data}
-
- Bernstein DI, Bateman ED, Woodcock A, Toler WT, Forth R, Jacques L, et al. Fluticasone furoate (FF)/vilanterol (100/25 mcg or 200/25 mcg) or FF (100 mcg) in persistent asthma. Journal of Asthma 2015;52(10):1073-83. - PubMed
Bodzenta‐Lukaszyk 2012 {published and unpublished data}
-
- Bodzenta-Lukaszyk A, Buhl R, Balint B, Lomax M, Spooner K, Dissanayake S. Fluticasone/formoterol combination therapy versus budesonide/formoterol for the treatment of asthma: a randomized, controlled, non-inferiority trial of efficacy and safety. Journal of Asthma 2012;49(10):1060-70. - PubMed
Busse 2008 {published data only}
-
- Busse WW, Shah SR, Somerville L, Parasuraman B, Martin P, Goldman M. Comparison of adjustable- and fixed-dose budesonide/formoterol pressurized metered-dose inhaler and fixed-dose fluticasone propionate/salmeterol dry powder inhaler in asthma patients. Journal of Allergy and Clinical Immunology 2008;121(6):1407-14. - PubMed
-
- O'Connor RD, Patrick DL, Parasuraman B, Martin P, Goldman M. Comparison of patient-reported outcomes during treatment with adjustable- and fixed-dose budesonide/formoterol pressurized metered-dose inhaler versus fixed-dose fluticasone propionate/salmeterol dry powder inhaler in patients with asthma. Journal of Asthma 2010;47(2):217-23. - PubMed
Cukier 2013 {published and unpublished data}
-
- Cukier A, Jacob CM, Rosario Filho NA, Fiterman J, Vianna EO, Hetzel JL, et al. Fluticasone/formoterol dry powder versus budesonide/formoterol in adults and adolescents with uncontrolled or partly controlled asthma. Respiratory Medicine 2013;107(9):1330-8. - PubMed
Gessner 2020 {published data only}
-
- Gessner C, Kornmann O, Maspero J, Zyl-Smit R, Krüll M, Salina A, et al. Fixed-dose combination of indacaterol/glycopyrronium/mometasone furoate once-daily versus salmeterol/fluticasone twice-daily plus tiotropium once-daily in patients with uncontrolled asthma: a randomised, phase IIIb, non-inferiority study (ARGON). Respiratory Medicine 2020;170:106021. - PubMed
Kerstjens 2012 {published data only}
-
- Kerstjens HA, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, et al. Tiotropium in asthma poorly controlled with standard combination therapy. New England Journal of Medicine 2012;367(13):1198-207. - PubMed
Kerstjens 2012a {published data only}
-
- Kerstjens HA, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, et al. Tiotropium in asthma poorly controlled with standard combination therapy. New England Journal of Medicine 2012;367(13):1198-207. - PubMed
Kerstjens 2012b {published data only}
-
- Kerstjens HA, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, et al. Tiotropium in asthma poorly controlled with standard combination therapy. New England Journal of Medicine 2012;367(13):1198-207. - PubMed
Kerstjens 2020 {published data only}
-
- Kerstjens HA, Maspero J, Chapman KR, Zyl-Smit RN, Hosoe M, Tanase AM, et al. Once-daily, single-inhaler mometasone-indacaterol-glycopyrronium versus mometasone-indacaterol or twice-daily fluticasone-salmeterol in patients with inadequately controlled asthma (IRIDIUM): a randomised, double-blind, controlled phase 3 study. Lancet Respiratory Medicine 2020;8(10):1000-12. - PubMed
Lee 2020 {published data only}
-
- Lee LA, Bailes Z, Barnes N, Boulet LP, Edwards D, Fowler A, et al. Efficacy and safety of once-daily single-inhaler triple therapy (FF/UMEC/VI) versus FF/VI in patients with inadequately controlled asthma (CAPTAIN): a double-blind, randomised, phase 3A trial. Lancet Respiratory Medicine 2021;9(1):69-84. - PubMed
Mansfield 2017 {published data only}
-
- Mansfield L, Yiu G, Sakov A, Liu S, Caracta C. A 6-month safety and efficacy study of fluticasone propionate and fluticasone propionate/salmeterol multidose dry powder inhalers in persistent asthma. Allergy and Asthma Proceedings 2017;38(4):264-76. - PubMed
-
- Miller DS, Yiu G, Hellriegel ET, Steinfeld J. Dose-ranging study of salmeterol using a novel fluticasone propionate/salmeterol multidose dry powder inhaler in patients with persistent asthma. Allergy and Asthma Proceedings 2016;37(4):291-301. - PubMed
Papi 2007 {published data only}
-
- Papi A, Paggiaro P, Nicolini G, Vignola AM, Fabbri LM. Beclomethasone/formoterol vs fluticasone/salmeterol inhaled combination in moderate to severe asthma. Allergy 2007;62(10):1182-8. - PubMed
Peters 2008 {published data only}
-
- Peters SP, Prenner BM, Mezzanotte WS, Martin P, O'Brien CD. Long-term safety and asthma control with budesonide/formoterol versus budesonide pressurized metered-dose inhaler in asthma patients. Allergy and Asthma Proceedings 2008;29(5):499-516. - PubMed
Stempel 2016 {published data only}
-
- Stempel DA, Raphiou IH, Kral KM, Yeakey AM, Emmett AH, Prazma CM, et al. Serious asthma events with fluticasone plus salmeterol versus fluticasone alone. New England Journal of Medicine 2016;374(19):1822-30. - PubMed
van Zyl‐Smit 2020 {published data only}
-
- Zyl-Smit RN, Krüll M, Gessner C, Gon Y, Noga O, Richard A. Once-daily mometasone plus indacaterol versus mometasone or twice-daily fluticasone plus salmeterol in patients with inadequately controlled asthma (PALLADIUM): a randomised, double-blind, triple-dummy, controlled phase 3 study. Lancet Respiratory Medicine 2020;8(10):987-99. - PubMed
Virchow 2019 {published data only}
-
- Virchow JC, Kuna P, Paggiaro P, Papi A, Singh D, Corre S, et al. Single inhaler extrafine triple therapy in uncontrolled asthma (TRIMARAN and TRIGGER): two double-blind, parallel-group, randomised, controlled phase 3 trials. Lancet 2019;394(10210):1737-49. - PubMed
Virchow 2019a {published data only}
-
- Virchow JC, Kuna P, Paggiaro P, Papi A, Singh D, Corre S, et al. Single inhaler extrafine triple therapy in uncontrolled asthma (TRIMARAN and TRIGGER): two double-blind, parallel-group, randomised, controlled phase 3 trials. Lancet 2019;394(10210):1737-49. - PubMed
Virchow 2019b {published data only}
-
- Virchow JC, Kuna P, Paggiaro P, Papi A, Singh D, Corre S, et al. Single inhaler extrafine triple therapy in uncontrolled asthma (TRIMARAN and TRIGGER): two double-blind, parallel-group, randomised, controlled phase 3 trials. Lancet 2019;394(10210):1737-49. - PubMed
Weinstein 2010 {published and unpublished data}
-
- Weinstein SF, Corren J, Murphy K, Nolte H, White M. Twelve-week efficacy and safety study of mometasone furoate/formoterol 200/10 microg and 400/10 microg combination treatments in patients with persistent asthma previously receiving high-dose inhaled corticosteroids. Allergy and Asthma Proceedings 2010;31(4):280-9. - PubMed
Woodcock 2013 {published data only}
-
- Woodcock A, Bleecker ER, Lötvall J, O'Byrne PM, Bateman ED, Medley H, et al. Efficacy and safety of fluticasone furoate/vilanterol compared with fluticasone propionate/salmeterol combination in adult and adolescent patients with persistent asthma: a randomized trial. Chest 2013;144(4):1222-9. - PMC - PubMed
References to studies excluded from this review
Akpinarli 1999 {published data only}
Allbers 2010 {published data only}
-
- Aalbers R, Backer V, Kava TT, Omenaas ER, Sandström T, Jorup C, et al. Adjustable maintenance dosing with budesonide/formoterol compared with fixed-dose salmeterol/fluticasone in moderate to severe asthma. Current Medical Research & Opinion 2004;20(2):225-40. - PubMed
-
- Aalbers R. Fixed or adjustable maintenance-dose budesonide/formoterol compared with fixed maintenance-dose salmeterol/fluticasone propionate in asthma patients aged >or=16 years: post hoc analysis of a randomized, double-blind/open-label extension, parallel-group study. Clinical Drug Investigation 2010;30(7):439-51. - PubMed
Antilla 2014 {published data only}
Aubier 1999 {published data only}
-
- Aubier M, Pieters WR, Schlösser NJ, Steinmetz KO. Salmeterol/fluticasone propionate (50/500 microg) in combination in a Diskus inhaler (Seretide) is effective and safe in the treatment of steroid-dependent asthma. Respiratory Medicine 1999;93(12):876-84. [PMID: PMID: 10653049] - PubMed
Bailey 2008 {published data only}
-
- Bailey W, Castro M, Matz J, White M, Dransfield M, Yancey S, et al. Asthma exacerbations in African Americans treated for 1 year with combination fluticasone propionate and salmeterol or fluticasone propionate alone. Current Medical Research & Opinion 2008;24(6):1669-82. [PMID: PMID: 18462564] - PubMed
Balki 2018 {published data only}
-
- Balki A, Balamurugan S, Bardapurkar S, Dalal S, Singh A, Singh BP, et al. Comparison of fluticasone/formoterol with budesonide/formoterol pMDI in adults with moderate to severe persistent asthma: results from a 12-week randomized controlled trial. Pulmonary Pharmacology & Therapeutics 2018;48:28-36. [PMID: PMID: 28890299] - PubMed
Barnes 2013 {published data only}
-
- Barnes N, Noord JA, Brindicci C, Lindemann L, Varoli G, Perpiña M, et al. Stepping-across controlled asthmatic patients to extrafine beclometasone/formoterol combination. Pulmonary Pharmacology & Therapeutics 2013;26(5):555-61. - PubMed
Bateman 2011 {published data only}
-
- Bateman ED, Kornmann O, Schmidt P, Pivovarova A, Engel M, Fabbri LM. Tiotropium is noninferior to salmeterol in maintaining improved lung function in B16-Arg/Arg patients with asthma. Journal of Allergy & Clinical Immunology 2011;128(2):315-22. - PubMed
Bateman 2014 {published data only}
Beasley 2015 {published data only}
Bernstein 2018 {published data only}
-
- Bernstein D, Andersen L, Forth R, Jacques L, Yates L. Once-daily fluticasone furoate/vilanterol versus twice-daily fluticasone propionate/salmeterol in patients with asthma well controlled on ICS/LABA. Journal of Asthma 2018;55(9):984-93. - PubMed
Blais 2016 {published data only}
-
- Blais CM, Davis BE, Cockcroft DW. Duration of bronchoprotection of the long-acting muscarinic antagonists tiotropium & glycopyrronium against methacholine-induced bronchoconstriction in mild asthmatics. Respiratory Medicine 2016;118:96-101. [PMID: PMID: 27578477] - PubMed
Blais 2017 {published data only}
Bleecker 2012 {published data only}
-
- Bleecker ER, Bateman ED, Busse WW, Woodcock A, Frith L, House KW, et al. Once-daily fluticasone furoate is efficacious in patients with symptomatic asthma on low-dose inhaled corticosteroids. Annals of Allergy, Asthma & Immunology 2012;109(5):353-8. [PMID: PMID: 23062392] - PubMed
Bodzenta‐Lukaszyk 2011 {published data only}
-
- Bodzenta-Lukaszyk A, Dymek A, McAulay K, Mansikka H. Fluticasone/formoterol combination therapy is as effective as fluticasone/salmeterol in the treatment of asthma, but has a more rapid onset of action: an open-label, randomized study. BMC Pulmonary Medicine 2011;11:28. [PMID: PMID: 21605396] - PMC - PubMed
Boyd 1995 {published data only}
-
- Boyd G. Salmeterol xinafoate in asthmatic patients under consideration for maintenance oral corticosteroid therapy. UK Study Group. European Respiratory Journal 1995;8(9):1494-8. [PMID: PMID: 8575574] - PubMed
Buhl 2003 {published data only}
-
- Buhl R, Creemers JP, Vondra V, Martelli NA, Naya IP, Ekström T. Once-daily budesonide/formoterol in a single inhaler in adults with moderate persistent asthma. Respiratory Medicine 2003;97(4):323-30. [PMID: PMID: 12693793] - PubMed
Busse 2013 {published data only}
-
- Busse WW, O'Byrne PM, Bleecker ER, Lötvall J, Woodcock A, Andersen L, et al. Safety and tolerability of the novel inhaled corticosteroid fluticasone furoate in combination with the β2 agonist vilanterol administered once daily for 52 weeks in patients >=12 years old with asthma: a randomised trial. Thorax 2013;68(6):513-20. - PMC - PubMed
Dahl 2006 {published data only}
-
- Dahl R, Engel M, Dusser D, Halpin D, Kerstjens HA, Zaremba-Pechmann L, et al. Safety and tolerability of once-daily tiotropium Respimat(®) as add-on to at least inhaled corticosteroids in adult patients with symptomatic asthma: A pooled safety analysis. Respiratory Medicine 2016;118:102-11. [PMID: PMID: 27578478] - PubMed
Devillier 2018 {published data only}
-
- Devillier P, Humbert M, Boye A, Zachgo W, Jacques L, Nunn C, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (FF/VI) versus twice-daily inhaled corticosteroids/long-acting β 2-agonists (ICS/LABA) in patients with uncontrolled asthma: An open-label, randomized, controlled trial. Respiratory Medicine 2018;141:111-20. [PMID: PMID: 30053956] - PubMed
D’Urzo 2001 {published data only}
-
- D'Urzo AD, Chapman KR, Cartier A, Hargreave FE, Fitzgerald M, Tesarowski D. Effectiveness and safety of salmeterol in nonspecialist practice settings. Chest 2001;119(3):714-9. [PMID: PMID: 11243947] - PubMed
EUCTR2008‐004833‐70 {unpublished data only}
-
- EUCTR2008-004833-70. A phase iii, randomized, parallel group, open study to compare the therapeutic efficacy and safety of SMB budesonide-salmeterol DPI capsule 150/25 µg bid delivered by the Axahaler versus Symbicort Turbuhaler 200/12 µg bid over 12 weeks in moderate to severe persistent asthmatic patients. https://www.clinicaltrialsregister.eu/ctr-search/search?query=2008-00483... (first received 19 January 2009).
Fitzgerald 1999 {published data only}
-
- FitzGerald JM, Chapman KR, Della Cioppa G, Stubbing D, Fairbarn MS, Till MD, et al. Sustained bronchoprotection, bronchodilatation, and symptom control during regular formoterol use in asthma of moderate or greater severity. Journal of Allergy and Clinical Immunology 1999;103:427--35. [PMID: PMID: 10069876] - PubMed
Gardiner 1994 {published data only}
-
- Gardiner PV, Ward C, Booth H, Allison A, Hendrick DJ, Walters EH. Effect of eight weeks of treatment with salmeterol on bronchoalveolar lavage inflammatory indices in asthmatics. American Journal of Respiratory and Critical Care Medicine 1994;150(4):1006-11. [PMID: PMID: 7921429] - PubMed
Godard 2008 {published data only}
-
- Godard P, Greillier P, Pigearias B, Nachbaur G, Desfougeres JL, Attali V. Maintaining asthma control in persistent asthma: comparison of three strategies in a 6-month double-blind randomised study. Respiratory Medicine 2008;102(8):1124-31. [PMID: PMID: 18606533] - PubMed
Green 2006 {published data only}
-
- Green RH, Brightling CE, McKenna S, Hargadon B, Neale N, Parker D, et al. Comparison of asthma treatment given in addition to inhaled corticosteroids on airway inflammation and responsiveness. European Respiratory Journal 2006;27(6):1144-51. [PMID: PMID: 16455831] - PubMed
Hamelmann 2016 {published data only}
-
- Hamelmann E, Bateman ED, Vogelberg C, Szefler SJ, Vandewalker M, Moroni-Zentgraf P, et al. Tiotropium add-on therapy in adolescents with moderate asthma: A 1-year randomized controlled trial. Journal of Allergy and Clinical Immunology 2016;138(2):441-50. - PubMed
Hamelmann 2017 {published data only}
Hoshino 2016 {published data only}
-
- Hoshino M, Ohtawa J, Akitsu K. Effects of the addition of tiotropium on airway dimensions in symptomatic asthma. Allergy and Asthma Proceedings 2016;37(6):147-53. [PMID: PMID: 27931291] - PubMed
Houghton 2007 {published data only}
Hultquist 2000 {published data only}
-
- Hultquist C, Domeij W, Kasak V, Laitinen L, O'Neil S. Oxis turbuhaler (formoterol), Accolate (zafirlukast) or placebo as add‐on treatment to Pulmicort turbuhaler (budesonide) in asthmatic patients on inhaled steroids. Astra Zeneca Report No: SD‐4004CR‐02162000 2000.
Ind 2003 {published data only}
-
- Ind PW, Dal Negro R, Colman NC, Fletcher CP, Browning D, James MH. Addition of salmeterol to fluticasone propionate treatment in moderate-to-severe asthma. Respiratory Medicine 2003;97(5):555-62. [PMID: PMID: 12735675] - PubMed
Ishiura 2018 {published data only}
-
- Ishiura Y, Fujimura M, Shiba Y, Ohkura N, Hara J, Abo M, et al. A comparison of the efficacy of once-daily fluticasone furoate/vilanterole with twice-daily fluticasone propionate/salmeterol in elderly asthmatics. Drug Research 2018;68(1):38-44. [PMID: ] - PubMed
Katial 2011 {published data only}
-
- Katial RK, Bernstein D, Prazma CM, Lincourt WR, Stempel DA. Long-term treatment with fluticasone propionate/salmeterol via Diskus improves asthma control versus fluticasone propionate alone. Allergy and Asthma Proceedings 2011;32(2):127-36. [PMID: PMID: 21189151] - PubMed
Kerstjens 2015 {published data only}
-
- Kerstjens HA, Casale TB, Bleecker ER, Meltzer EO, Pizzichini E, Schmidt O, et al. Tiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trials. Lancet Respiratory Medicine 2015;3(5):367-76. - PubMed
Kerwin 2009 {published data only}
-
- Kerwin EM, Oppenheimer JJ, LaForce C, Parasuraman B, Miller CJ, O'Dowd L, et al. Efficacy and tolerability of once-daily budesonide/formoterol pressurized metered-dose inhaler in adults and adolescents with asthma previously stable with twice-daily budesonide/ formoterol dosing. Annals of Allergy, Asthma and Immunology 2009;103(1):62-72. [PMID: PMID: 19663129] - PubMed
Kerwin 2011 {published data only}
-
- Kerwin E, Prazma CM, Sutton L, Stempel DA. Safety and efficacy of long-term treatment with fluticasone propionate and salmeterol via DISKUS versus fluticasone propionate alone. Clinical Research and Regulatory Affairs 2011;28:14-21. [DOI: 10.3109/10601333.2010.544315] - DOI
Kerwin 2021 {published data only}
-
- Kerwin E, Dorinsky P, Patel M, Rossman K, Reisner C, Maes A, et al. A randomized controlled trial of glycopyrrolate administered by metered dose inhaler in patients with uncontrolled asthma despite ICS/LABA treatment. Journal of Asthma 2021;Aug 1:1-13. [DOI: 10.1080/02770903.2021.1938603] [PMID: PMID: 34338132] - DOI - PubMed
-
- NCT03358147. Efficacy and safety of PT001 to placebo and open-label Spiriva® Respimat® in subjects with persistant asthma. clinicaltrials.gov/ct2/show/NCT03358147 (first received 30 November 2017).
Koenig 2008 {published data only}
-
- Koenig SM, Murray JJ, Wolfe J, Andersen L, Yancey S, Prillaman B, et al. Does measuring BHR add to guideline derived clinical measures in determining treatment for patients with persistent asthma? Respiratory Medicine 2008;102(5):665-73. [PMID: PMID: 18328683] - PubMed
Kuna 2007 {published data only}
Kupczyk 2021 {published data only}
-
- Kupczyk M, Majak P, Kuna P, Asankowicz-Bargiel B, Barańska E, Dobek R. A new formulation of fluticasone propionate/salmeterol in a metered-dose inhaler (MDI HFA) allows for the reduction of a daily dose of corticosteroid and provides optimal asthma control - A randomized, multi-center, non-inferiority, phase IV clinical study. Respiratory Medicine 2021 Jan;176:106274. [DOI: 10.1016/j.rmed.2020.106274] [PMID: ] - DOI - PubMed
Langton Hewer 1995 {published data only}
-
- Langton Hewer S, Hobbs J, French D, Lenney W. Pilgrim's progress: the effect of salmeterol in older children with chronic severe asthma. Respiratory Medicine 1995;89(6):435-40. [PMID: PMID: 7644775] - PubMed
Lee 2015 {published data only}
-
- Lee LA, Yang S, Kerwin E, Trivedi R, Edwards LD, Pascoe S, et al. The effect of fluticasone furoate/umeclidinium in adult patients with asthma: a randomized, dose-ranging study. Respiratory Medicine 2015;109(1):54-62. [PMID: PMID: 25452139] - PubMed
Lenney 2013 {published data only}
-
- Lenney W, McKay AJ, Tudur Smith C, Williamson PR, James M, Price D. Management of asthma in school age children on therapy (MASCOT): a randomised, double-blind, placebo-controlled, parallel study of efficacy and safety. Health Technology Assessment 2013;17(4):1-218. [PMID: PMID: 23380178] - PMC - PubMed
Li 2010 {published data only}
-
- Li JS, Qaqundah PY, Weinstein SF, LaForce CF, Ellsworth AV, Ortega HG, et al. Fluticasone propionate/salmeterol combination in children with asthma: key cardiac and overall safety results. Clinical Research and Regulatory Affairs 2010;27(3):87-95.
Lin 2015 {published data only}
-
- Lin J, Kang J, Lee SH, Wang C, Zhou X, Crawford J, et al. Fluticasone furoate/vilanterol 200/25 mcg in Asian asthma patients: a randomized trial. Respiratory Medicine 2015;109(1):44-53. [PMID: PMID: 25524507] - PubMed
Lotvall 2014 {published data only}
-
- Lötvall J, Bateman ED, Busse WW, O'Byrne PM, Woodcock A, Toler WT, et al. Comparison of vilanterol, a novel long-acting beta2 agonist, with placebo and a salmeterol reference arm in asthma uncontrolled by inhaled corticosteroids. Journal of Negative Results in Biomedicine 2014;13(1):9. [PMID: PMID: 24928338] - PMC - PubMed
Malone 2005 {published data only}
-
- Malone R, LaForce C, Nimmagadda S, Schoaf L, House K, Ellsworth A, et al. The safety of twice-daily treatment with fluticasone propionate and salmeterol in pediatric patients with persistent asthma. Annals of Allergy, Asthma and Immunology 2005;95(1):66-71. [PMID: PMID: 16095144] - PubMed
Maspero 2010 {published data only}
Maspero 2014 {published data only}
-
- Maspero J, Cherrez I, Doherty DE, Tashkin DP, Kuna P, Kuo WL, et al. Appraisal of lens opacity with mometasone furoate/formoterol fumarate combination in patients with COPD or asthma. Respiratory Medicine 2014;108(9):1355-62. [PMID: PMID: 25044280] - PubMed
Meijer 1995 {published data only}
-
- Meijer GG, Postma DS, Mulder PG, Aalderen WM. Long-term circadian effects of salmeterol in asthmatic children treated with inhaled corticosteroids. American Journal of Respiratory and Critical Care Medicine 1995;152:1887-92. [PMID: PMID: 8520751] - PubMed
Morice 2008 {published data only}
-
- Morice AH, Peterson S, Beckman O, Kukova Z. Efficacy and safety of a new pressurised metered-dose inhaler formulation of budesonide/formoterol in children with asthma: a superiority and therapeutic equivalence study. Pulmonary Pharmacology and Therapeutics 2008;21(1):152-9. [PMID: PMID: 17376722] - PubMed
Muraki 2013 {published data only}
-
- Muraki M, Soutome T, Hashimoto K, Tohda Y. Long-term study of fluticasone furoate/vilanterol combination (FF/VI) and FF alone in Japanese adult patients with bronchial asthma. Allergologia et Immunopathologia 2013;20:110-25.
NCT00118690 {unpublished data only}
-
- NCT00118690. A study measuring asthma control in pediatric and adolescent subjects whose asthma is worsened by activity or exercise. clinicaltrials.gov/ct2/show/NCT00118690 (first received 12 July 2005).
NCT00118716 {unpublished data only}
-
- NCT00118716. A study measuring asthma control in pediatric and adolescent subjects whose asthma is worsened by activity or exercise. clinicaltrials.gov/ct2/show/NCT00118716 (first received 12 July 2005).
NCT01192178 {unpublished data only}
-
- NCT01192178. Fall epidemic viral pediatric study. clinicaltrials.gov/ct2/show/NCT01192178 (first received 31 August 2010).
NCT01570478 {unpublished data only}
-
- NCT01570478. A study in patients with asthma (NELSON). clinicaltrials.gov/ct2/show/NCT01570478 (first received 4 April 2012).
NCT02127697 {published data only}
-
- NCT02127697. Study of efficacy and safety of NVA237 in patients with poorly controlled asthma. clinicaltrials.gov/ct2/show/NCT02127697 (first received 1 May 2014).
NCT02296411 {published data only}
-
- NCT02296411. Efficacy of LAMA added to ICS in treatment of asthma (ELITRA). www.clinicaltrials.gov/ct2/show/NCT02296411 (first received 20 November 2014).
NCT02433834 {unpublished data only}
-
- NCT02433834. Chronic dosing cross-over study to assess the efficacy and safety of glycopyrronium (PT001) in adult subjects with intermittent asthma or mild to moderate persistent asthma. clinicaltrials.gov/ct2/show/NCT02433834 (first received 5 May 2015).
NCT02892344 {unpublished data only}
-
- NCT02892344. Study of QMF149 (150/80 µg) compared with MF Twisthaler® (200 µg) in patients with asthma. clinicaltrials.gov/ct2/show/NCT02892344 (first received 8 September 2016).
NCT03063086 {published data only}
-
- NCT03063086. Assess bronchodilator effect and safety of two doses of QVM149 compared to a fixed dose combination of salmeterol/fluticasone in patients with asthma. clinicaltrials.gov/ct2/show/NCT03063086 (first received 24 February 2017).
NCT03184987 {unpublished data only}
-
- NCT03184987. A long-term safety study of fixed dose combination therapy fluticasone furoate/umeclidinium bromide/vilanterol trifenatate in Japanese subjects with asthma. clinicaltrials.gov/ct2/show/NCT03184987 (first received 14 June 2017).
NCT03376932 {unpublished data only}
-
- NCT03376932. Effectiveness of fluticasone furoate/ umeclidinium/ vilanterol (FF/UMEC/VI) using the connected inhaler system (CIS) as compared with fluticasone proprionate/ salmeterol (FP/SAL) plus tiotropium (TIO) in inadequately controlled asthma. clinicaltrials.gov/ct2/show/NCT03376932 (first received 19 December 2017).
Norhaya 1999 {published data only}
-
- Norhaya MR, Yap TM, Zainudin BM. Addition of inhaled salmeterol to inhaled corticosteroids in patients with poorly controlled nocturnal asthma. Respirology 1999;4(1):77-81. [PMID: PMID: 10339734] - PubMed
O'Byrne 2014 {published data only}
O'Byrne 2016 {published data only}
Ohta 2015 {published data only}
-
- Ohta K, Ichinose M, Tohda Y, Engel M, Moroni-Zentgraf P, Kunimitsu S, et al. Long-term once-daily tiotropium Respimat® is well tolerated and maintains efficacy over 52 weeks in patients with symptomatic asthma in Japan: a randomised, placebo-controlled study. PLOS One 2015;10(4):e0124109. - PMC - PubMed
Paggiaro 2016 {published data only}
-
- Paggiaro P, Halpin DM, Buhl R, Engel M, Zubek VB, Blahova Z, et al. The effect of Tiotropium in symptomatic asthma despite low- to medium-dose inhaled corticosteroids: a randomized controlled trial. Journal of Allergy and Clinical Immunology. In Practice 2016 Jan-Feb;4(1):104-13. - PubMed
Peters 2010 {published data only}
Peters 2016 {published data only}
-
- Peters SP, Bleecker ER, Canonica GW, Park YB, Ramirez R, Hollis S, et al. Serious asthma events with budesonide plus formoterol vs. budesonide alone. New England Journal of Medicine 2016;375(9):850-60. [PMID: PMID: 27579635] - PubMed
Ploszczuk 2018 {published data only}
Pohunek 2006 {published data only}
-
- Pohunek P, Kuna P, Jorup C, De Boeck K. Budesonide/formoterol improves lung function compared with budesonide alone in children with asthma. Pediatric Allergy and Immunology 2006;17(6):458-65. [PMID: PMID: 16925692] - PubMed
Price 2002 {published data only}
-
- Price D, Haughney J, Duerden M, Nicholls C, Moseley C. The cost effectiveness of chlorofluorocarbon-free beclomethasone dipropionate in the treatment of chronic asthma: a cost model based on a 1-year pragmatic, randomised clinical study. Pharmacoeconomics 2002;20(10):653-64. [PMID: PMID: 12162754] - PubMed
Rajanandh 2014 {published data only}
-
- Rajanandh MG, Nageswari AD, Ilango K. Assessment of various second-line medications in addition to inhaled corticosteroid in asthma patients: a randomized controlled trial. Clinical and Experimental Pharmacology and Physiology 2014;41(7):509-13. - PubMed
-
- Rajanandh MG, Nageswari AD, Ilango K. Pulmonary function assessment in mild to moderate persistent asthma patients receiving montelukast, doxofylline, and tiotropium with budesonide: a randomized controlled study. Clinical Therapeutics 2014;36(4):526-33. - PubMed
Raphael 2017 {published data only}
-
- Raphael G, Yiu G, Sakov A, Liu S, Caracta C. Randomized, double-blind trial evaluating the efficacy and safety of fluticasone propionate and fluticasone propionate/salmeterol delivered via multidose dry powder inhalers in patients with persistent asthma aged 12 years and older. Journal of Asthma 2018;55(6):640-50. - PubMed
Reddel 2007 {published data only}
-
- Reddel HK, Peyters MJ, Wark PA, Sand IB, Jenkins CR. Comparison of the efficacy of seretide and flixotide when down‐titrating the inhaled corticosteroid dose. Respirology 2007;12(Suppl 1):A40.
Renzi 2010 {published data only}
-
- Renzi PM, Howard LA, Ortega HG, Ahmad FF, Chapman KR. Low-dose fluticasone propionate with and without salmeterol in steroid-naïve patients with mild, uncontrolled asthma. Respiratory Medicine 2010;104(4):510-7. - PubMed
Russell 1995 {published data only}
-
- Russell G, Williams DA, Weller P, Price JF. Salmeterol xinafoate in children on high dose inhaled steroids. Annals of Allergy, Asthma and Immunology 1995;75(5):423-8. [PMID: PMID: 7583864] - PubMed
Sher 2017 {published data only}
-
- Sher LD, Yiu G, Sakov A, Liu S, Caracta CF. Fluticasone propionate and fluticasone propionate/salmeterol multidose dry powder inhalers compared with placebo for persistent asthma. Allergy and Asthma Proceedings 2017;38(5):343-53. [PMID: PMID: 28639542] - PubMed
Simons 1997 {published data only}
-
- Simons FE. A comparison of beclomethasone, salmeterol, and placebo in children with asthma. Canadian beclomethasone dipropionate-salmeterol xinafoate study group. New England Journal of Medicine 1997;337(23):1659-65. [PMID: PMID: 9385125] - PubMed
Stelmach 2008 {published data only}
-
- Stelmach I, Grzelewski T, Majak P, Jerzynska J, Stelmach W, Kuna P. Effect of different antiasthmatic treatments on exercise-induced bronchoconstriction in children with asthma. Journal of Allergy and Clinical Immunology 2008;121(2):383-9. [PMID: PMID: 17980416] - PubMed
Stempel 2016x {published data only}
-
- Stempel DA, Szefler SJ, Pedersen S, Zeiger RS, Yeakey AM, Lee LA, et al. Safety of adding salmeterol to fluticasone propionate in children with asthma. New England Journal Medicine 2016;375(9):840-9. [PMID: PMID: 27579634] - PubMed
Svedsater 2018 {published data only}
-
- Svedsater H, Jones R, Bosanquet N, Jacques L, Lay-Flurrie J, Leather DA, et al. Patient-reported outcomes with initiation of fluticasone furoate/vilanterol versus continuing usual care in the Asthma Salford Lung Study. Respiratory Medicine 2018;141:198-206. [PMID: ] - PubMed
Tal 2002 {published data only}
-
- Tal A, Simon G, Vermeulen JH, Petru V, Cobos N, Everard ML, et al. Budesonide/formoterol in a single inhaler versus inhaled corticosteroids alone in the treatment of asthma. Pediatric Pulmonology 2002;34(5):342-50. [PMID: PMID: 12357478] - PubMed
Teper 2005 {published data only}
-
- Teper AM, Kofman CD, Szulman GA, Vidaurreta SM, Maffey AF. Fluticasone improves pulmonary function in children under 2 years old with risk factors for asthma. American Journal of Respiratory and Critical Care Medicine 2005;171(6):587-90. [PMID: PMID: 15591466] - PubMed
Verberne 1998 {published data only}
-
- Verberne AA, Frost C, Duiverman EJ, Grol MH, Kerrebijn KF. Addition of salmeterol versus doubling the dose of beclomethasone in children with asthma. The Dutch asthma study group. American Journal of Respiratory and Critical Care Medicine 1998;158(1):213-9. [PMID: PMID: 9655732] - PubMed
Watz 2019 {published and unpublished data}
-
- Watz H, Hohlfeld, JM, Singh D, Beier J, Scholz V, Diamant Z, et al. The combination of indacaterol/glycopyrronium/mometasone furoate is superior to high-dose salmeterol/fluticasone propionate in improving lung function in patients with asthma. In: American Journal of Respiratory and Critical Care Medicine. Vol. 199. 2019:A7081.
Wechsler 2016 {published data only}
-
- Wechsler ME, Yawn BP, Fuhlbrigge AL, Pace WD, Pencina MJ, Doros G, et al. Anticholinergic vs long-acting β-agonist in combination with inhaled corticosteroids in black adults with asthma: the BELT randomized clinical trial. JAMA 2015;314(16):1720-30. - PubMed
Weiler 2005 {published data only}
-
- Weiler JM, Nathan RA, Rupp NT, Kalberg CJ, Emmett A, Dorinsky PM. Effect of fluticasone/salmeterol administered via a single device on exercise-induced bronchospasm in patients with persistent asthma. Annals of Allergy, Asthma and Immunology 2005;94(1):65-72. [PMID: PMID: 15702819] - PubMed
Weinstein 2019 {published data only}
-
- Weinstein CL, Ryan N, Shekar T, Gates D, Lane SJ, Agache I, et al. Serious asthma events with mometasone furoate plus formoterol compared with mometasone furoate. Journal of Allergy and Clinical Immunology 2019;134(4):1395-402. - PubMed
Yang 2015 {published data only}
-
- Yang S, Goyal N, Beerahee M, Trivedi R, Lee L, Pascoe S. Dose-response modelling of umeclidinium and fluticasone furoate/umeclidinium in asthma. European Journal of Clinical Pharmacology 2015;71(9):1051-8. [PMID: PMID: 26174114] - PubMed
Zhang 2018 {published data only}
Zimmerman 2004 {published data only}
-
- Zimmerman B, D'Urzo A, Bérubé D. Efficacy and safety of formoterol Turbuhaler when added to inhaled corticosteroid treatment in children with asthma. Pediatric Pulmonology 2004;37(2):122-7. [PMID: PMID: 14730657] - PubMed
References to ongoing studies
NCT03387241 {published data only}
-
- NCT03387241. Efficacy of FLUTIFORM ® vs Seretide® in moderate to severe persistent asthma in subjects aged ≥12 years. clinicaltrials.gov/ct2/show/NCT03387241 (first received 2 January 2018).
NCT04191434 {published data only}
-
- NCT04191434. Efficacy and safety of flamboyant 125/12 association in the treatment of adults with moderate asthma. clinicaltrials.gov/ct2/show/NCT04191434 (first received 9 December 2019).
NCT04191447 {published data only}
-
- NCT04191447. Efficacy and safety of flamboyant 200/12 association in the treatment of adults with severe asthma. clinicaltrials.gov/ct2/show/NCT04191447 (first received 9 December 2019).
NCT04609878 {published data only}
-
- NCT04609878. Study to assess PT010 in adult and adolescent participants with inadequately controlled asthma (KALOS) (KALOS). clinicaltrials.gov/ct2/show/NCT04609878 (first received 30 October 2020).
NCT04609904 {published data only}
-
- NCT04609904. Study to assess PT010 in adult and adolescent participants with inadequately controlled asthma (LOGOS) (LOGOS). clinicaltrials.gov/ct2/show/NCT04609904 (first received 30 October 2020).
NCT04937387 {unpublished data only}
-
- NCT04937387. Efficacy and safety of fluticasone furoate/umeclidinium/vilanterol (ff/umec/vi) in chinese participants with inadequately controlled asthma. clinicaltrials.gov/ct2/show/NCT04937387 (first received 24 June 2021).
NCT05018598 {unpublished data only}
-
- NCT05018598. Step-up to medium strength triple therapy vs high strength ICS/LABA in adult asthmatics uncontrolled on medium strength ICS/LABA (MiSTIC). clinicaltrials.gov/ct2/show/NCT05018598 (first received 24 August 2021).
Additional references
Aalbers 2017
Anderson 2015
-
- Anderson DE, Kew KM, Boyter AC. Long-acting muscarinic antagonists (LAMA) added to inhaled corticosteroids (ICS) versus the same dose of ICS alone for adults with asthma. Cochrane Database of Systematic Reviews 2015, Issue 8. Art. No: CD011397. [DOI: 10.1002/14651858.CD011397.pub2] [PMID: ] - DOI - PMC - PubMed
Barnes 2010
Beasley 2019
Befekadu 2014
BTS/SIGN 2019
-
- BTS/SIGN British Guideline on the Management of Asthma 2019. www.brit-thoracic.org.uk/quality-improvement/guidelines/asthma/ 2019.
Buhl 2021
-
- Buhl R, Nikolaev I, Tillmann HC, Vaidya S, Bartels C, Jain M, et al. Dose bridging data for mometasone furoate in once-daily fixed-dose inhaled combinations of mometasone furoate/indacaterol and mometasone furoate/ indacaterol/glycopyrronium in patients with asthma. Pulmonary Pharmacology & Therapeutics 2021 Oct;70:102068. [DOI: 10.1016/j.pupt.2021.102068] [PMID: ] - DOI - PubMed
Cochrane Airways 2019
-
- Cochrane Airways Trials Register. airways.cochrane.org/trials-register (accessed 7 May 2019).
Covidence [Computer program]
-
- Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation, June 29, 2021. Available at www.covidence.org.
Dechartres 2013
Deeks 2020
-
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). The Cochrane Collaboration, 2020. Available from training.cochrane.org/handbook.
Derom 1992
Dias 2013a
Dias 2013b
Dias 2013c
Ducharme 2010
-
- Ducharme FM, Ni Choinin M, Greenstone I, Lasserson TJ. Addition of long-acting beta 2-agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database of Systematic Reviews 2010, Issue 4. Art. No: CD005533. [DOI: 10.1002/14651858.CD005533.pub2] [PMC4169792] [PMID: ] - DOI - PMC - PubMed
FDA 2016
-
- US Food and Drug Administration. What is a serious adverse event? www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-e... (accessed 26 October 2021).
GeMTC package [Computer program]
-
- gemtc. Valkenhoef G and Kuiper J, Version 1.0-1. Groningen, Netherlands: Gert van Valkenhoef, May 14, 2021. https://CRAN.R-project.org/package=gemtc.
GINA 2021
-
- Global Initiative for Asthma. 2021 GINA Report, Global Strategy for Asthma Management and Prevention. ginasthma.org/gina-reports/ (accessed 6 April 2022).
Global Asthma Report 2018
-
- Global Asthma Network. The Global Asthma Report 2018. globalasthmareport.org/Global%20Asthma%20Report%202018.pdf (accessed prior to 26 October 2020).
Gosens 2018
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 22 January 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Guyatt 2011a
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE Working Group. GRADE guidelines: 7. Rating the quality of evidence--inconsistency. Journal of Clinical Epidemiology 2011 Dec;64(12):1294-302. [PMID: ] - PubMed
Guyatt 2011b
Guyatt 2017
-
- Guyatt GH, Ebrahim S, Alonso-Coello P, Johnston BC, Mathioudakis AG, Briel M, et al. GRADE guidelines 17: assessing the risk of bias associated with missing participant outcome data in a body of evidence. Journal of Clinical Epidemiology 2017;87:14-22. [PMID: ] - PubMed
Haahtela 1991
-
- Haahtela T, Järvinen M, Kava T, Kiviranta K, Koskinen S, Lehtonen K, et al. Comparison of a beta 2-agonist, terbutaline, with an inhaled corticosteroid, budesonide, in newly detected asthma. New England Journal of Medicine 1991;325(6):388-92. [DOI: 10.1056/NEJM199108083250603] [PMID: ] - DOI - PubMed
Higgins 2019
-
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). The Cochrane Collaboration, 2019. Available from training.cochrane.org/handbook.
Juniper 1994
Juniper 2005
-
- Juniper EF, Svensson K, Mörk AC, Ståhl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respiratory Medicine 2005;99(5):553-8. [PMID: ] - PubMed
Juniper 2006
Kew 2016
Kim 2021
Kips 2001
Marshall 2018
McDonald 2017
-
- McDonald S, Noel-Storr AH, Thomas J. Harnessing the efficiencies of machine learning and Cochrane Crowd to identify randomised trials for individual Cochrane reviews. In: Global Evidence Summit; 2017 September 13-16; Cape Town, South Africa. 2017.
McIvor 2014
-
- McIvor RA. Respiratory disorders: chronic obstructive pulmonary disease. In: Gray J, editors(s). Therapeutic Choices. 7th edition. Ottawa (ON): Canadian Pharmaceutical Association, 2014.
Moher 2009
NICE 2018
-
- National Institute for Health and Care Excellence. Inhaled corticosteroid doses for NICE’s asthma guideline. www.nice.org.uk/guidance/ng80/resources/inhaled-corticosteroid-doses-pdf... (accessed prior to 27 October 2020).
Noel‐Storr 2018
-
- Noel-Storr AH, Project Transform team. Cochrane Crowd: new ways of working together to produce health evidence. In: Evidence Live; 2018 June 18-20; Oxford, UK. 2018.
Nüesch 2010
O'Byrne 2019
OpenBUGS [Computer program]
-
- OpenBUGS. Thomas A, Version 3.2.3. Helsinki: Andrew Thomas, 2010. www.openbugs.net/w/FrontPage.
Pandya 2014
Petsky 2018
Phillippo 2018
Phillippo 2019
-
- Phillippo DM, Dias S, Welton NJ, Caldwell DM, Taske N, Ades AE. Threshold analysis as an alternative to GRADE for assessing confidence in guideline recommendations based on network meta-analyses. Annals of Internal Medicine 2019;170(8):538-46. [DOI: 10.7326/M18-3542] [PMC6739230] [PMID: ] - DOI - PMC - PubMed
R [Computer program]
-
- R: A Language and Environment for Statistical Computing. R Core Team, Version 4.1.1. Vienna, Austria: R Foundation for Statistical Computing, 2021-08-10. https://www.R-project.org.
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
RoB 2 Excel tool [Computer program]
-
- Risk of Bias 2 tool. London, England: Cochrane, 22 August 2019. Available at https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool/curren....
Rogliani 2021
Röver 2021
-
- Röver C, Bender R, Dias S, Schmid CH, Schmidli H, Sturtz S, et al. On weakly informative prior distributions for the heterogeneity parameter in Bayesian random‐effects meta‐analysis. Research Synthesis Methods 2021;12(4):448-74. - PubMed
Schatz 2006
-
- Schatz M, Sorkness CA, Li JT, Marcus P, Murray JJ, Nathan RA, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. Journal of Allergy and Clinical Immunology 2006;117(3):549-56. [DOI: 10.1016/j.jaci.2006.01.011] [PMID: ] - DOI - PubMed
Schünemann 2020
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). The Cochrane Collaboration, 2020. Available from training.cochrane.org/handbook.
Sears 2014
Shimoda 2016
Sobieraj 2018a
-
- Sobieraj DM, Baker WL, Nguyen E, Weeda ER, Coleman CI, White CM, et al. Association of inhaled corticosteroids and long-acting muscarinic antagonists with asthma control in patients with uncontrolled, persistent asthma: a systematic review and meta-analysis. JAMA 2018;319(14):1473-84. [DOI: 10.1001/jama.2018.2757] [PMID: ] - DOI - PMC - PubMed
Sobieraj 2018b
-
- Sobieraj DM, Weeda ER, Nguyen E, Coleman CI, White CM, Lazarus SC, et al. Association of inhaled corticosteroids and long-acting beta 2-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma: a systematic review and meta-analysis. JAMA 2018;319(14):1485-96. [DOI: 10.1001/jama.2018.2769] [PMID: ] - DOI - PMC - PubMed
Soriano 2017
-
- GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respiratory Medicine 2017;5(9):691-706. [DOI: 10.1016/S2213-2600(17)30293-X. Epub 2017 Aug 16] [PMID: ] - PMC - PubMed
Spiegelhalter 2002
-
- Spiegelhalter DJ, Best NG, Carlin BP, Van der Linde A. Bayesian measures of model complexity and fit. Journal of the Royal Statistical Society Series B (Statistical Methodology) 2002;64(4):583-616.
Sterne 2019
Thomas 2017
-
- Thomas J, Noel-Storr AH, Marshall I, Wallace B, McDonald S, Mavergames C, et al. Living systematic review network. Living systematic reviews: 2. Combining human and machine effort. Journal of Clinical Epidemiology 2017;91:31-7. - PubMed
Turner 2015
Vaidya 2016
-
- Vaidya SS, Khindri S, Calder N, Machineni S, Hara H, Majumdar T, et al. Pharmacokinetics of indacaterol and mometasone furoate delivered alone or in a free or fixed dose combination in healthy subjects. Pulmonary Pharmacology and Therapeutics 2016;37:30-6. [DOI: 10.1016/j.pupt.2016.01.004] [PMID: ] - DOI - PubMed
van Valkenhoef 2016
Yepes‐Nuñez 2019
References to other published versions of this review
Oba 2020
-
- Oba Y, Maduke T, Anwer S, Patel T, Dias S. Effectiveness and tolerability of dual and triple combination inhaler therapies compared with each other and varying doses of inhaled corticosteroids in adolescents and adults with asthma: a systematic review and network meta-analysis. Cochrane Database of Systematic Reviews November 24, 2020, Issue 11. Art. No: CD013799. [DOI: 10.1002/14651858.CD013799] - DOI - PMC - PubMed

