Gut colonization by Bacteroides requires translation by an EF-G paralog lacking GTPase activity
- PMID: 36472247
- PMCID: PMC9841332
- DOI: 10.15252/embj.2022112372
Gut colonization by Bacteroides requires translation by an EF-G paralog lacking GTPase activity
Abstract
Protein synthesis is crucial for cell growth and survival yet one of the most energy-consuming cellular processes. How, then, do cells sustain protein synthesis under starvation conditions when energy is limited? To accelerate the translocation of mRNA-tRNAs through the ribosome, bacterial elongation factor G (EF-G) hydrolyzes energy-rich guanosine triphosphate (GTP) for every amino acid incorporated into a protein. Here, we identify an EF-G paralog-EF-G2-that supports translocation without hydrolyzing GTP in the gut commensal bacterium Bacteroides thetaiotaomicron. EF-G2's singular ability to sustain protein synthesis, albeit at slow rates, is crucial for bacterial gut colonization. EF-G2 is ~10-fold more abundant than canonical EF-G1 in bacteria harvested from murine ceca and, unlike EF-G1, specifically accumulates during carbon starvation. Moreover, we uncover a 26-residue region unique to EF-G2 that is essential for protein synthesis, EF-G2 dissociation from the ribosome, and responsible for the absence of GTPase activity. Our findings reveal how cells curb energy consumption while maintaining protein synthesis to advance fitness in nutrient-fluctuating environments.
Keywords: Bacteroides thetaiotaomicron; GTP hydrolysis; elongation factor G; paralogous proteins; ribosome.
© 2022 The Authors.
Figures

- A
Alignment of the deduced amino acid sequences of EF‐G1 (EF‐G), EF‐G2, spdEF‐G1 and spdEF‐G2 proteins. spdEF‐G1 spdEF‐G2 are EF‐G paralogs found only in
S pirochaetes,P lanctomycetes andD elta‐proteobacteria, which are the only bacteria that do not harbor EF‐G orthologs (Margus et al, 2011). Borrelia burgdorferi (Bbu) spdEF‐G1 is only active in translocation but not ribosome recycling; by contrast, Bbu spdEF‐G2 was found to function specifically in ribosome recycling without translocation activity (Suematsu et al, 2010). Black triangle indicates the position of S84 in B. thetaiotaomicron EF‐G2 and H91 in E. coli EF‐G. Black diamonds indicate the conserved glutamine in loop I of domain IV, including Q507 in E. coli EF‐G and Q491 in B. thetaiotaomicron EF‐G2, and conserved histidine in loop II of domain IV, including H583 in E. coli EF‐G and H593 in B. thetaiotaomicron EF‐G2. Msm, Mycobacterium smegmatis; Tth, T. thermophilus; Bbu, Borrelia burgdorferi; Bth, B. thetaiotaomicron; Eco, E. coli; Pae, Pseudomonas aeruginosa. - B
Alignment of the deduced amino acid sequences of EF‐G2 proteins from the Bacteroidetes phylum showing the S84 region and the region including the Bacteroidetes EF‐G2‐specific insert.
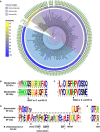
- A
Phylogenetic tree of 149 whole‐genome sequenced strains from the Bacteroidetes phylum, including 71 Bacteroides spp. strains (light blue background), with the blue‐yellow heatmap showing the presence of EF‐G1 or EF‐G2 sequelog, and the identity between each homolog and the corresponding B. thetaiotaomicron VPI‐5482 protein; the ring of letters (aa84) shows amino acid at the position 84 (B. thetaiotaomicron EF‐G2 numbering) of corresponding EF‐G2 proteins; and the outermost bar‐charts [Insert (bp)] shows the length of the insert corresponding to residues 514–539 in B. thetaiotaomicron EF‐G2. Background colors: blue—Bacteroides genus; purple—Bacteroidaceae family; gray—Bacteroidales order, Bacteroidia class. The three listed Bacteroidetes lacking EF‐G2 sequelogs are: Flavobacterium johnsoniae, which is a soil bacterium; Candidatus Azobacteroides pseudotrichonymphae, which was isolated from a single cell of the protist Pseudotrichonympha grassii, which resides in the termite gut; Porphyromonas sp. KLE 1280, which is a human oral bacterium.
- B, C
Sequence logos of the aligned deduced amino acid sequences of the EF‐G1 and EF‐G2 proteins from Bacteroides species showing the regions corresponding to Loop I (B) and Loop II (C) at the tip of domain IV in canonical EF‐G. Only unique (non‐redundant) protein sequences were used for the alignment.
- D
Sequence logos of aligned deduced amino acid sequences of EF‐G1 and EF‐G2 proteins from Bacteroides species showing the region surrounding the EF‐G2 insert with amino acids that form beta‐strand and beta‐hairpin in B. thetaiotaomicron EF‐G2 shown by arrows below the logos.
- E
Sequence logos of aligned deduced amino acid sequences of EF‐G1 and EF‐G2 proteins from Bacteroides species showing the G3‐box (DTPG in canonical EF‐G) followed by the conserved histidine in Bacteroides EF‐G1s and the corresponding serine/alanine in Bacteroides EF‐G2s. The B. thetaiotaomicron EF‐G2 has a serine at this position (S84).

- A
Western blot analysis of reporter HslO‐FLAG protein produced with a custom‐made PURExpress® coupled in vitro transcription‐translation system supplemented with the indicated EF‐G proteins and incubated for the indicated times. Blot was developed using anti‐FLAG antibodies. Shown is a representative from at least two independent experiments. Eco EF‐G: E. coli EF‐G.
- B
Ribosome translocation determined as the fluorescence change of the fluoresceine‐labeled mRNA in stopped‐flow experiment supplemented with the indicated proteins and nucleotides. Translocation rates are as follows: EF‐G1 with GTP, 6.9 ± 0.1 s−1; EF‐G1 with GDPNP, 0.64 9 ± 0.01 s−1; EF‐G2 with GTP, 0.7 ± 0.01 s−1; EF‐G2 with GDPNP, 0.62 ± 0.01 s−1. See Materials and Methods for details. Shown are averages of 5–7 technical replicates.
- C
Tripeptide formation assay using translation initiation complex programed with the tripeptide‐encoding mRNA, corresponding aminoacyl‐tRNA:EF‐Tu:GTP ternary complexes and GTP, and the indicated EF‐G proteins following incubation for 1 or 5 min. Shown are the results from two independent experiments and their average. Insert: tripeptide formation with EF‐G2 following incubation for 0, 1, 5, and 10 min.

- A
Ribosome translocation measured by monitoring the fluorescence change of the fluoresceine‐labeled mRNA in a stopped‐flow experiment supplemented with the indicated proteins. Translocation rates are as follows: E. coli EF‐G, 8.4 ± 0.1 s−1; EF‐G1, 8.3 ± 0.2 s−1; EF‐G2, 0.7 ± 0.1 s−1. See Materials and Methods for details. Shown are averages of 5–7 technical replicates.
- B
Ribosome translocation determined by monitoring the fluorescence change of the fluoresceine‐labeled mRNA in a stopped‐flow experiment supplemented with the indicated proteins and nucleotides. See Materials and Methods for details. Shown are averages of 5–7 technical replicates.
- C
Western blot analysis of polysome fractions prepared from strain WH407, which expresses EF‐G1‐FLAG and EF‐G2‐HA from their normal chromosomal locations and promoters. Blots were developed using anti‐FLAG and anti‐HA antibodies.
- D
Ribosome splitting effect of EF‐G1, EF‐G2, or E. coli EF‐G in the presence of RRF from the same species were measured by light scattering in a stopped‐flow apparatus. See Materials and Methods for details.
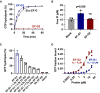
- A
GTP hydrolysis exhibited by the indicated EF‐G proteins in the presence of pre‐translocation ribosomes. Shown are the results from three independent experiments.
- B
GTP hydrolysis exhibited by the indicated EF‐G proteins in the presence of a B. thetaiotaomicron ribosome preparation. Shown are the results from three technical replicates and their average, error bars correspond to SD. P‐values are from two‐tailed Student's t‐test between each protein and the blank, ns indicate P > 0.05.
- C
GTP hydrolysis by EF‐G1 (1 μM) measured in the presence of a fixed amount of vacant E. coli ribosomes and varied amounts of EF‐G2 (1–10 μM, 1×–10×). Shown are the results from three independent experiments and their average, error bars correspond to SD.
- D
GTP binding by EF‐G1 and EF‐G2 measured using differential radial capillary action of ligand assay (DraCALA; Roelofs et al, 2011). Shown are results of three technical replicates. The DraCALA blot of one representative replicate is shown in Fig EV3C. See Materials and Methods for details.

- A
GTP hydrolysis exhibited by the indicated EF‐G proteins in the presence of vacant E. coli ribosomes. Mean values and SD for three independent experiments are shown.
- B
No GTP hydrolysis is exhibited by the EF‐G1 and EF‐G2 proteins in the absence of ribosomes.
- C
GTP binding of the EF‐G1 and EF‐G2 proteins (at the indicated concentrations) by differential radial capillary action of ligand assay (DraCALA; Roelofs et al, 2011). A representative from three replicates is shown.
- D
Binding of radioactive GTP to the EF‐G1 and EF‐G2 proteins in the presence of the indicated concentrations non‐radioactive (cold) GTP or GDP (assayed via DraCALA). A representative from three replicates is shown.

- A
Structure of EF‐G2 domain IV highlighting the insert (residues 514–539) and hairpin (residues 516–527) regions.
- B
Western blot analysis of reporter HslO‐FLAG protein synthesized in vitro using a custom‐made PURExpress® system with EF‐G1, EF‐G2, and two engineered EF‐G2 variants: one missing the 26‐amino acid insert, EF‐G2 (∆514–539) and one missing the beta hairpin in the insert, EF‐G2 (∆516–527). Shown is a representative experiment from at least two independent experiments.
- C
GTP hydrolysis by EF‐G1, EF‐G2 and indicated variants incubated in the presence of vacant E. coli ribosomes. Shown are results from three technical replicates and their average, error bars correspond to SD, and P‐values derived from two‐tailed Student's t‐test comparing each protein to the blank.
- D
SDS–PAGE analysis of co‐sedimentation assays monitoring factor (EF‐G1 EF‐G2 or EF‐G2 variant EF‐G2 (∆516–527) or EF‐G2 (∆514–539)) binding to E. coli 70 S ribosomes. Binding reactions were centrifuged through a sucrose cushion to recover ribosome and associated factor in the pellets. The positions of EF‐G proteins and ribosomal proteins and molecular weight markers are indicated.
- E
Western blot analysis of reporter HisC‐FLAG protein synthesized in vitro using a custom‐made PURExpress® system with the EF‐G1, EF‐G2 or EF‐G2 (S84H) proteins. Shown is a representative from at least two independent experiments.

- A
Overview of Class 1 (left) and Class 2 (right) structures derived from workflow shown in Appendix Fig S1. The degrees of inter‐subunit rotation and 30S head swiveling (as compared to PDB: 7ST6) are shown below 30S subunit head (dark slate gray) and body (light slate gray). 50S subunit is shown in light blue, EF‐G2 in red and EF‐G2 insert in dark blue.
- B
Close‐up view of the EF‐G2 insert region in domain IV in Class 1 (left) and Class 2 (right) structures. Same color scheme as in panel (A).

- A
Overview of the EF‐G2‐ribosome complex structure. The ribosomal 50S subunit is in gray and the 30S subunit in light blue. The color of the five domains of EF‐G2 are as follows: blue—I, pink—II, orange—III, green—IV, light purple—V. The same color scheme for the EF‐G2 domains is used in other panels.
- B
Overview of the five‐domain structure of the EF‐G2 protein.
- C
Comparison of the EF‐G2 structure with that of E. coli EF‐G (PDB: 7N2V) in light gray. The structures are aligned by their domain I.
- D
Comparison of domain IV of EF‐G2 and E. coli EF‐G (PDB: 7N2V) in light gray, highlighting the 26‐amino acid insert present in EF‐G2 (residues 514–539) and absent from EF‐G1 and E. coli EF‐G (Fig EV1).
- E
Local density and model fitting of the resolved switch I and II regions and GTP in the ligand‐binding pocket of EF‐G2. The switch I (residues K37‐L67) region is colored orange, the switch II (D80‐V97) region blue, the P‐loop (G16‐T23) purple and the G4‐box (N134‐D137) region green. The same color scheme is used in other panels for EF‐G2.
- F
Fitting GTP or GDP‐Pi in the density of the ligand showing GTP fits better. The models of T. thermophilus EF‐G2‐GTP (PDB: 2DY1) or E. coli EF‐G‐GDP‐Pi (PDB: 7SSL) were rigid‐body fitted in focused refined EF‐G2 map, and density corresponding to the ligand were extracted as shown in the upper and lower panels, respectively.
- G
Superimposed structures of the GTP‐binding pocket including the switch I and II regions of EF‐G2 and the GTP‐bound conformation of E. coli EF‐G (PDB: 7N2V, light gray) and T. thermophilus EF‐G2 (PDB: 2DY1, dark gray).
- H, I
Superimposed structures showing position of the ribosomal sarcin‐ricin loop (SRL, yellow) relative to EF‐G2 (colored) or GDPCP‐bound E. coli EF‐G on vacant ribosome (PDB: 4V9O) (dark gray) (H), and GDP‐bound E. coli EF‐G on chimeric translocation intermediate (PDB: 7SSD) (light gray) (I). Structures were aligned by the 23S rRNA. Red arrows indicate the rotation of EF‐G2 domains I and II relative to E. coli EF‐G structures. Same color scheme for EF‐G2 as in previous panels.
- J
Superimposed structures showing position of the SRL (yellow) relative to the GTP binding pocket of EF‐G2 (colored) or GDPCP‐bound E. coli EF‐G on vacant ribosome (PDB: 4V9O) (light gray).
- K
Distance (in Å) between SRL (Cα of A2662) and GTP‐binding pocket (Cα of T23 in the P‐loop and Pβ of the ligand GTP/analog/GDP/GDP‐Pi) in the EF‐G2 structures and in published structures of ribosome‐bound E. coli or T. thermophilus EF‐G with PDB identifiers indicated in parenthesis.
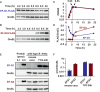
- A–D
Western blot analysis of B. thetaiotaomicron strains expressing C‐terminally FLAG‐tagged EF‐G1 (WH405) (A), or EF‐G2 (GT1301) (B), from their normal promoters and chromosomal locations, grown in Tryptone Yeast Extract Glucose liquid medium (TYG) and sampled at the indicated times. Bacterial growth (OD600) is shown in (C) and Western blot quantifications (FLAG signal normalized to loading control GroEL) are show in (D). Blots were developed with anti‐FLAG antibodies; anti‐GroEL antibodies were used as loading controls. Shown is a representative from at least two independent experiments.
- E, F
Western blot of crude extracts from wild‐type B. thetaiotaomicron harvested from the ceca of mice (representative blot for two out of four mice tested) or corresponding to stationary phase cultures harvested following growth in TYG for 24 h or purified EF‐G1 and EF‐G2 proteins at the indicated amounts (E). Blots were developed with anti‐EF‐G1 or EF‐G2 polyclonal antibodies; anti‐GroEL antibodies were used as loading controls. Western blot quantifications of all four biological replicates and their average are shown in (F), where EF‐G1 and EF‐G2 abundances were estimated by normalizing the signal of bacterial sample to the closest signal of known amount of purified protein, and the derived absolute amount (pmol) was normalized to the signal of GroEL. Error bars represent SD. Please note log scale of y axis.

- A–C
Western blot analysis of crude extracts prepared from (B). thetaiotaomicron strains encoding a C‐terminal FLAG‐tagged EF‐G1 (WH405) (A), or a C‐terminal FLAG‐tagged EF‐G2 (GT1301) (B), expressed from their normal promoters and chromosomal locations during exponential growth in minimal medium with glucose as carbon source (MM, time point: −5 min), and after shifted to the same minimal medium (MM) or to carbon‐ (No C) or nitrogen‐ (No N) limited minimal media for 15 or 60 min. Blots were developed with anti‐FLAG antibodies; anti‐GroEL antibodies were used as loading controls. A representative biological replicate from duplicates is shown. Western blot quantifications (FLAG signal normalized to GroEL signal) for the biological duplicates and their averages are shown in (C).

References
-
- Belardinelli R, Sharma H, Caliskan N, Cunha CE, Peske F, Wintermeyer W, Rodnina MV (2016) Choreography of molecular movements during ribosome progression along mRNA. Nat Struct Mol Biol 23: 342–348 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

