Biomarker response to high-specific-activity I-131 meta-iodobenzylguanidine in pheochromocytoma/paraganglioma
- PMID: 36472300
- PMCID: PMC9874967
- DOI: 10.1530/ERC-22-0236
Biomarker response to high-specific-activity I-131 meta-iodobenzylguanidine in pheochromocytoma/paraganglioma
Abstract
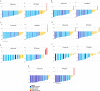
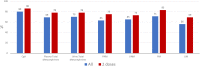
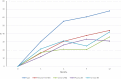
The objective of this study is to present the complete biomarker response dataset from a pivotal trial evaluating the efficacy and safety of high-specific-activity I-131 meta-iodobenzylguanidine in patients with advanced pheochromocytoma or paraganglioma. Biomarker status was assessed and post-treatment responses were analyzed for catecholamines, metanephrines, and serum chromogranin A. Complete biomarker response (normalization) or partial response, defined as at least 50% reduction from baseline if above the normal range, was evaluated at specified time points over a 12-month period. These results were correlated with two other study objectives: blood pressure control and objective tumor response as per RECIST 1.0. In this open-label, single-arm study, 68 patients received at least one therapeutic dose (~18.5 GBq (~500 mCi)) of high-specific-activity I-131 meta-iodobenzylguanidine. Of the patients, 79% and 72% had tumors associated with elevated total plasma free metanephrines and serum chromogranin A levels, respectively. Best overall biomarker responses (complete or partial response) for total plasma free metanephrines and chromogranin A were observed in 69% (37/54) and 80% (39/49) of patients, respectively. The best response for individual biomarkers was observed 6-12 months following the first administration of high-specific-activity I-131 meta-iodobenzylguanidine. Biochemical tumor marker response was significantly associated with both reduction in antihypertensive medication use (correlation coefficient 0.35; P = 0.006) as well as objective tumor response (correlation coefficient 0.36; P = 0.007). Treatment with high-specific-activity I-131 meta-iodobenzylguanidine resulted in long-lasting biomarker responses in patients with advanced pheochromocytoma or paraganglioma that correlated with blood pressure control and objective response rate. ClinicalTrials.gov number: NCT00874614.
Keywords: biomarker response; high-specific-activity I-131 MIBG; paraganglioma; pheochromocytoma.
Conflict of interest statement
NS and VAD are employees of Progenics Pharmaceuticals, Inc., a Lantheus company. CJ, BBC, RBN, JSD, LS and DAP served as investigators for this clinical study.
Figures
References
-
- Amar L, Servais A, Gimenez-Roqueplo AP, Zinzindohoue F, Chatellier G, Plouin PF.2005Year of diagnosis, features at presentation, and risk of recurrence in patients with pheochromocytoma or secreting paraganglioma. Journal of Clinical Endocrinology and Metabolism 902110–2116. (10.1210/jc.2004-1398) - DOI - PubMed
-
- Ayala-Ramirez M, Feng L, Habra MA, Rich T, Dickson PV, Perrier N, Phan A, Waguespack S, Patel S, Jimenez C.2012Clinical benefits of systemic chemotherapy for patients with metastatic pheochromocytomas or sympathetic extra-adrenal paragangliomas: insights from the largest single-institutional experience. Cancer 1182804–2812. (10.1002/cncr.26577) - DOI - PMC - PubMed
-
- Ayala-Ramirez M, Feng L, Johnson MM, Ejaz S, Habra MA, Rich T, Busaidy N, Cote GJ, Perrier N, Phan Aet al.2011Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: primary tumor size and primary tumor location as prognostic indicators. Journal of Clinical Endocrinology and Metabolism 96717–725. (10.1210/jc.2010-1946) - DOI - PubMed
-
- AZEDRA®2021. Full prescribing information | AZEDRA® (iobenguane | 131). Billercia, MA, USA: Progenics Pharmaceuticals Inc., a Lantheus Company. (available at: https://www.azedra.com/content/pdf/full-prescribing-information.pdf)
-
- Barrett JA, Joyal JL, Hillier SM, Maresca KP, Femia FJ, Kronauge JF, Boyd M, Mairs RJ, Babich JW.2010Comparison of high-specific-activity ultratrace 123/131I-MIBG and carrier-added 123/131I-MIBG on efficacy, pharmacokinetics, and tissue distribution. Cancer Biotherapy and Radiopharmaceuticals 25299–308. (10.1089/cbr.2009.0695) - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

