Efficacy of a Small-Molecule Inhibitor of KrasG12D in Immunocompetent Models of Pancreatic Cancer
- PMID: 36472553
- PMCID: PMC9900321
- DOI: 10.1158/2159-8290.CD-22-1066
Efficacy of a Small-Molecule Inhibitor of KrasG12D in Immunocompetent Models of Pancreatic Cancer
Abstract
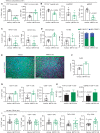
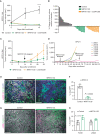
Mutations in the KRAS oncogene are found in more than 90% of patients with pancreatic ductal adenocarcinoma (PDAC), with Gly-to-Asp mutations (KRASG12D) being the most common. Here, we tested the efficacy of a small-molecule KRASG12D inhibitor, MRTX1133, in implantable and autochthonous PDAC models with an intact immune system. In vitro studies validated the specificity and potency of MRTX1133. In vivo, MRTX1133 prompted deep tumor regressions in all models tested, including complete or near-complete remissions after 14 days. Concomitant with tumor cell apoptosis and proliferative arrest, drug treatment led to marked shifts in the tumor microenvironment (TME), including changes in fibroblasts, matrix, and macrophages. T cells were necessary for MRTX1133's full antitumor effect, and T-cell depletion accelerated tumor regrowth after therapy. These results validate the specificity, potency, and efficacy of MRTX1133 in immunocompetent KRASG12D-mutant PDAC models, providing a rationale for clinical testing and a platform for further investigation of combination therapies.
Significance: Pharmacologic inhibition of KRASG12D in pancreatic cancer models with an intact immune system stimulates specific, potent, and durable tumor regressions. In the absence of overt toxicity, these results suggest that this and similar inhibitors should be tested as potential, high-impact novel therapies for patients with PDAC. See related commentary by Redding and Grabocka, p. 260. This article is highlighted in the In This Issue feature, p. 247.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures


Comment in
-
A Splendid New Beginning at the End of a 40-Year Quest: The First KRASG12D Inhibitor in Pancreatic Cancer.Cancer Discov. 2023 Feb 6;13(2):260-262. doi: 10.1158/2159-8290.CD-22-1304. Cancer Discov. 2023. PMID: 36744321
References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7–33. - PubMed
-
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. . FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–25. - PubMed
-
- Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, et al. . FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med 2018;379:2395–406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

