Long-Term Effectiveness and Drug Survival of Secukinumab in Vietnamese Patients with Psoriasis: Results from a Retrospective ENHANCE Study
- PMID: 36472791
- PMCID: PMC9884729
- DOI: 10.1007/s13555-022-00867-y
Long-Term Effectiveness and Drug Survival of Secukinumab in Vietnamese Patients with Psoriasis: Results from a Retrospective ENHANCE Study
Erratum in
-
Correction to: Long-Term Effectiveness and Drug Survival of Secukinumab in Vietnamese Patients with Psoriasis: Results from a Retrospective ENHANCE Study.Dermatol Ther (Heidelb). 2023 Nov;13(11):2927. doi: 10.1007/s13555-023-01021-y. Dermatol Ther (Heidelb). 2023. PMID: 37661246 Free PMC article. No abstract available.
Abstract
Introduction: Psoriasis (PsO), an immune-mediated inflammatory skin disorder, has substantial negative impact on patients' quality of life. Secukinumab, an approved treatment for moderate-to-severe plaque PsO, has an established long-term efficacy and safety profile. This study aims to provide real-world evidence of long-term effectiveness and retention rate of secukinumab in Vietnamese patients with PsO.
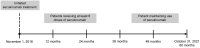
Methods: This retrospective, observational study collected medical records of adult patients with moderate-to-severe PsO receiving secukinumab treatment from Ho Chi Minh City Hospital of Dermato-Venereology. The primary objective was to evaluate secukinumab effectiveness in PsO as measured by 75% improvement in psoriasis area and severity index (PASI 75) at month 12. Secondary objectives were PASI 90/100, absolute PASI ≤ 3 and ≤ 5, Dermatology Life Quality Index (DLQI), and retention rate over 48 months.
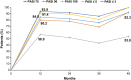
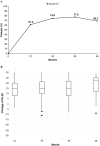
Results: In total, 232 patients with moderate-to-severe PsO met inclusion criteria; 68.1% were male, with median age and age of onset of 39 and 27.5 years, respectively. Median time from onset of PsO to secukinumab treatment was 120 months, 95.3% were prior biologics/disease-modifying antirheumatic drugs naive and 41.4% received concomitant therapies for PsO; 82.3% had national insurance coverage. At month 12, 93.9% of patients achieved PASI 75 (primary endpoint); 80.2/56.9% achieved PASI 90/100; 91.4 and 84.8% patients achieved absolute PASI ≤ 5 and ≤ 3, respectively. The response was sustained over 48 months, with 91.9%/78.0%/52.0% of patients achieving PASI 75/90/100, 89.5% and 82.1% patients achieving absolute PASI ≤ 5 and ≤ 3, respectively. At month 12, 61.4% of patients achieved DLQI 0/1 which was sustained up to month 48 (69.2%). Secukinumab adherence rate of 84.9% at month 12 dropped to 34.2% at month 48. Patients receiving concomitant therapy and national insurance showed higher adherence rate.
Conclusion: Secukinumab demonstrated long-term effectiveness in real-world Vietnamese patients with moderate-to-severe PsO, with treatment adherence being higher in patients having concomitant therapies and national insurance.
Keywords: Biologics; Effectiveness; Psoriasis; Real-world; Secukinumab.
© 2022. The Author(s).
Conflict of interest statement
Hao T. Nguyen has served as an advisory board member and speaker for Novartis, Janssen, and Menarini. Nhi T. U. Pham, Tu N. A. Tran, and Thao T. P. Vu have served as a speaker for Novartis, Janssen, and Menarini. Yen T. Bui is an employee of Novartis Vietnam Co., Ltd. Nguyen N. Pham has nothing to disclose.
Figures


References
-
- Thaci D, Korber A, von Kiedrowski R, Bachhuber T, Melzer N, Kasparek T, et al. Secukinumab is effective in treatment of moderate-to-severe plaque psoriasis: real-life effectiveness and safety from the PROSPECT study. J Eur Acad Dermatol Venereol. 2020;34(2):310–318. doi: 10.1111/jdv.15962. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

