Effectiveness of Pfizer-BioNTech COVID-19 vaccine as evidence for policy action: A rapid systematic review and meta-analysis of non-randomized studies
- PMID: 36473010
- PMCID: PMC9725157
- DOI: 10.1371/journal.pone.0278624
Effectiveness of Pfizer-BioNTech COVID-19 vaccine as evidence for policy action: A rapid systematic review and meta-analysis of non-randomized studies
Abstract
In December 2020, an interim recommendation for the use of Pfizer-BioNTech COVID-19 vaccine in persons aged ≥16 years was made under Food and Drug Administration's Emergency Use Authorization. In preparation for Biologics License Application approval, we conducted a systematic review and meta-analysis to inform the U.S. Centers for Disease Control and Prevention's Advisory Committee for Immunization Practice's (ACIP) decision-making for a standard recommendation. We conducted a rapid systematic review and meta-analysis of Pfizer-BioNTech vaccine effectiveness (VE) against symptomatic COVID-19, hospitalization due to COVID-19, death due to COVID-19, and asymptomatic SARS-CoV-2 infection. We identified studies through August 20, 2021 from an ongoing systematic review conducted by the International Vaccine Access Center and the World Health Organization. We evaluated each study for risk of bias using the Newcastle-Ottawa Scale. Pooled estimates were calculated using meta-analysis. The body of evidence for each outcome was assessed using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach. We identified 80 articles, selected 35 for full-text review, and included 26. The pooled VE of Pfizer-BioNTech COVID-19 vaccine was 92.4% (95% CI: 87.5%-95.3%) against symptomatic COVID-19 with moderate evidence certainty (eight studies), 94.3% (95% CI: 87.9%-97.3%) against hospitalization due to COVID-19 with moderate certainty (eight studies), 96.1% (95% CI: 91.5%-98.2%) against death due to COVID-19 with moderate certainty (four studies), and 89.3% (88.4%-90.1%) against asymptomatic SARS-CoV-2 infection with very low certainty (two studies). The Pfizer-BioNTech COVID-19 vaccine demonstrated high effectiveness in all pre-specified outcomes and extended knowledge of the vaccine's benefits to outcomes and populations not informed by the RCTs. Use of an existing systematic review facilitated a rapid meta-analysis to inform an ACIP policy decision. This approach can be utilized as additional COVID-19 vaccines are considered for standard recommendations by ACIP.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
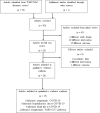


References
-
- Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, et al.. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69(50):1922–4. Epub 2020/12/18. doi: 10.15585/mmwr.mm6950e2 ; PubMed Central PMCID: PMC7745957 Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed. - DOI - PMC - PubMed
-
- Food and Drug Administration. Pfizer-BioNTech COVID-19 Vaccine Emergency Use Authorization. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2020. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise...
-
- Hannah Ritchie EM, Lucas Rodés-Guirao, Cameron Appel, Charlie Giattino, Esteban Ortiz-Ospina, Joe Hasell, et al. Coronavirus (COVID-19) Vaccinations Published online at OurWorldInData.org2020 [cited 2021 October 6]. Available from: https://ourworldindata.org/covid-vaccinations.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

