Relative Impact of Pain and Disease Activity on Improvements in Fatigue: Results From 2 Baricitinib Phase 3 Clinical Trials
- PMID: 36473106
- PMCID: PMC10045960
- DOI: 10.1097/RHU.0000000000001924
Relative Impact of Pain and Disease Activity on Improvements in Fatigue: Results From 2 Baricitinib Phase 3 Clinical Trials
Abstract
Background/objective: Fatigue is common in patients with rheumatoid arthritis (RA). We assessed the relative impact of pain and disease activity on improvements in fatigue in 2 phase 3 baricitinib clinical trials.
Methods: RA-BEAM (NCT01710358) and RA-BEACON (NCT01721044) were randomized, double-blind, placebo-controlled studies in adults with moderate to severe RA. RA-BEAM assessed baricitinib + methotrexate (MTX) and adalimumab + MTX in patients with prior inadequate response/intolerance (IR) to MTX (MTX-IR). RA-BEACON assessed patients with IR to ≥1 biologic disease-modifying antirheumatic drug (bDMARD-IR). Measures included the Functional Assessment of Chronic Illness Therapy-Fatigue scale, Clinical Disease Activity Index (CDAI) for RA, and pain visual analog scale (VAS). Analyses were implemented separately for each study.
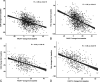
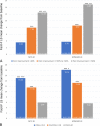
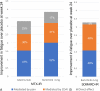
Results: Significant improvements were seen in disease activity and pain, which were greater with baricitinib versus adalimumab. A statistically significant improvement was seen in fatigue with both active treatments versus placebo. Moderate correlations were observed between improvements in disease activity and fatigue and between improvements in pain and fatigue in both MTX-IR and bDMARD-IR patients. Reductions in pain (≥50%) and remission or low disease activity (CDAI ≤10) had significant associations with fatigue improvement at week 24. In mediation analysis, improvements in fatigue attributable to CDAI and pain VAS in MTX-IR patients were 31% and 52%, respectively, for baricitinib, and 30% and 47%, respectively, for adalimumab. In bDMARD-IR patients, improvement in fatigue was attributed 48% to CDAI and 48% to pain VAS.
Conclusions: In both MTX-IR and bDMARD-IR patients, a large proportion of improvements in fatigue across treatment arms were accounted for by improvements in pain and disease activity.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc.
Figures
Similar articles
-
Patient-reported outcomes from a phase 3 study of baricitinib versus placebo or adalimumab in rheumatoid arthritis: secondary analyses from the RA-BEAM study.Ann Rheum Dis. 2017 Nov;76(11):1853-1861. doi: 10.1136/annrheumdis-2017-211259. Epub 2017 Aug 10. Ann Rheum Dis. 2017. PMID: 28798049 Free PMC article. Clinical Trial.
-
Efficacy of baricitinib in patients with moderate-to-severe rheumatoid arthritis up to 6.5 years of treatment: results of a long-term study.Rheumatology (Oxford). 2024 Oct 1;63(10):2799-2809. doi: 10.1093/rheumatology/keae012. Rheumatology (Oxford). 2024. PMID: 38258434 Free PMC article. Clinical Trial.
-
Patient-reported outcomes of baricitinib in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment.Arthritis Res Ther. 2017 Sep 18;19(1):208. doi: 10.1186/s13075-017-1410-1. Arthritis Res Ther. 2017. PMID: 28923098 Free PMC article. Clinical Trial.
-
Tofacitinib for Treating Rheumatoid Arthritis After the Failure of Disease-Modifying Anti-rheumatic Drugs: An Evidence Review Group Perspective of a NICE Single Technology Appraisal.Pharmacoeconomics. 2018 Sep;36(9):1063-1072. doi: 10.1007/s40273-018-0639-0. Pharmacoeconomics. 2018. PMID: 29546668 Review.
-
Baricitinib for Previously Treated Moderate or Severe Rheumatoid Arthritis: An Evidence Review Group Perspective of a NICE Single Technology Appraisal.Pharmacoeconomics. 2018 Jul;36(7):769-778. doi: 10.1007/s40273-018-0616-7. Pharmacoeconomics. 2018. PMID: 29502174 Free PMC article. Review.
Cited by
-
Potential Mechanism of Fatigue Induction and Its Management by JAK Inhibitors in Inflammatory Rheumatic Diseases.J Inflamm Res. 2023 Sep 8;16:3949-3965. doi: 10.2147/JIR.S414739. eCollection 2023. J Inflamm Res. 2023. PMID: 37706062 Free PMC article. Review.
-
Integrated bioinformatics analysis of the effects of chronic pain on patients with spinal cord injury.Front Cell Neurosci. 2025 Feb 5;19:1457740. doi: 10.3389/fncel.2025.1457740. eCollection 2025. Front Cell Neurosci. 2025. PMID: 39974584 Free PMC article.
References
-
- Kirwan JR Minnock P Adebajo A, et al. . Patient perspective: fatigue as a recommended patient centered outcome measure in rheumatoid arthritis. J Rheumatol. 2007;34:1174–1177. - PubMed
-
- Nikolaus S Bode C Taal E, et al. . Fatigue and factors related to fatigue in rheumatoid arthritis: a systematic review. Arthritis Care Res (Hoboken). 2013;65:1128–1146. - PubMed
-
- Hewlett S Chalder T Choy E, et al. . Fatigue in rheumatoid arthritis: time for a conceptual model. Rheumatology (Oxford). 2011;50:1004–1006. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

