Stimuli-Responsive Membrane Anchor Peptide Nanofoils for Tunable Membrane Association and Lipid Bilayer Fusion
- PMID: 36473125
- PMCID: PMC9782321
- DOI: 10.1021/acsami.2c11946
Stimuli-Responsive Membrane Anchor Peptide Nanofoils for Tunable Membrane Association and Lipid Bilayer Fusion
Abstract
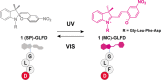
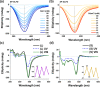
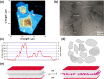
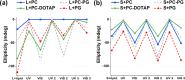
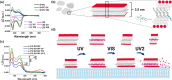
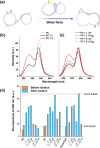
Self-assembled peptide nanostructures with stimuli-responsive features are promising as functional materials. Despite extensive research efforts, water-soluble supramolecular constructs that can interact with lipid membranes in a controllable way are still challenging to achieve. Here, we have employed a short membrane anchor protein motif (GLFD) and coupled it to a spiropyran photoswitch. Under physiological conditions, these conjugates assemble into ∼3.5 nm thick, foil-like peptide bilayer morphologies. Photoisomerization from the closed spiro (SP) form to the open merocyanine (MC) form of the photoswitch triggers rearrangements within the foils. This results in substantial changes in their membrane-binding properties, which also varies sensitively to lipid composition, ranging from reversible nanofoil reformation to stepwise membrane adsorption. The formed peptide layers in the assembly are also able to attach to various liposomes with different surface charges, enabling the fusion of their lipid bilayers. Here, SP-to-MC conversion can be used both to trigger and to modulate the liposome fusion efficiency.
Keywords: lipid bilayer fusion; liposomes; membrane activity; peptide bilayer; self-assembly; spiropyran.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Photo-responsive liposomes composed of spiropyran-containing triazole-phosphatidylcholine: investigation of merocyanine-stacking effects on liposome-fiber assembly-transition.Soft Matter. 2019 May 8;15(18):3740-3750. doi: 10.1039/c8sm02181c. Soft Matter. 2019. PMID: 31042253
-
On the microscopic and mesoscopic perturbations of lipid bilayers upon interaction with the MPER domain of the HIV glycoprotein gp41.Biochim Biophys Acta. 2016 Aug;1858(8):1904-13. doi: 10.1016/j.bbamem.2016.05.007. Epub 2016 May 12. Biochim Biophys Acta. 2016. PMID: 27179640
-
Bilayer Thickness and Curvature Influence Binding and Insertion of a pHLIP Peptide.Biophys J. 2018 May 8;114(9):2107-2115. doi: 10.1016/j.bpj.2018.03.036. Biophys J. 2018. PMID: 29742404 Free PMC article.
-
DNA Oligonucleotide-Functionalized Liposomes: Bioconjugate Chemistry, Biointerfaces, and Applications.Langmuir. 2018 Dec 11;34(49):15000-15013. doi: 10.1021/acs.langmuir.8b01368. Epub 2018 Jul 9. Langmuir. 2018. PMID: 29936848 Review.
-
Tethered bilayer lipid membranes (tBLMs): interest and applications for biological membrane investigations.Biochimie. 2014 Dec;107 Pt A:135-42. doi: 10.1016/j.biochi.2014.06.021. Epub 2014 Jul 4. Biochimie. 2014. PMID: 24998327 Review.
Cited by
-
Lipophilic Anchors that Embed Bioconjugates in Bilayer Membranes: A Review.Bioconjug Chem. 2023 Jun 21;34(6):961-971. doi: 10.1021/acs.bioconjchem.3c00204. Epub 2023 Jun 5. Bioconjug Chem. 2023. PMID: 37276240 Free PMC article. Review.
-
Light-controlled smart materials: Supramolecular regulation and applications.Smart Mol. 2024 Sep 15;2(4):e20240036. doi: 10.1002/smo.20240036. eCollection 2024 Dec. Smart Mol. 2024. PMID: 40626274 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

