Cross-Sectional Analysis of Clinical Trial Availability and North Carolina Neighborhood Social Vulnerability
- PMID: 36473128
- PMCID: PMC9970296
- DOI: 10.1200/OP.22.00325
Cross-Sectional Analysis of Clinical Trial Availability and North Carolina Neighborhood Social Vulnerability
Abstract
Purpose: Residents of communities facing social vulnerability (eg, poverty) have limited access to clinical trials, leaving them susceptible to experiencing poor health outcomes. We examined the association between North Carolina county-level social vulnerability and available multiple myeloma (MM) trials.
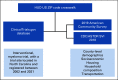
Methods: Using a novel data linkage between ClinicalTrials.gov, the 2019 American Community Survey, and the Centers for Disease Control and Prevention's Social Vulnerability Index, we investigated at the county level (1) availability of MM trial sites and (2) the relationship between Social Vulnerability Index and MM trial site availability using logistic regression.
Results: Between 2002 and 2021, 229 trials were registered across 462 nonunique trial sites in 34 counties. Nearly 50% of trial sites were in academic medical centers, 80% (n = 372) of all trials were industry-sponsored, 60% (n = 274) were early-phase, and 50% (n = 232) were for patients with relapsed or refractory MM. Counties with low as opposed to high poverty rates had six times greater odds of having ≥ 1 MM trial sites (odds ratio [OR], 5.60; 95% CI, 1.85 to 19.64; P = .004). Counties with the lowest percentage of Black Indigenous Persons of Color and non-native English speakers had 77% lower odds (OR, 0.23; 95% CI, 0.07 to 0.69; P = .011) of having ≥ 1 trial sites. The effect remained significant after accounting for the presence of five academic medical centers (n = 95; OR, 0.18; 95% CI, 0.05 to 0.6; P = .008) and adjustment for metropolitan, suburban, or rural status (OR, 0.25; 95% CI, 0.07 to 0.81; P = .025).
Conclusion: Counties with the lowest poverty rates had more MM trial sites, whereas those with the lowest percentage of Black Indigenous Persons of Color populations had fewer MM trial sites. Multilevel efforts are needed to improve the availability and access to trials for socially vulnerable populations.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures


References
-
- SEER*Explorer: An Interactive Website for SEER Cancer Statistics. Surveillance Research Program and National Cancer Institute, Research Program, National Cancer Institute. https://seer.cancer.gov/explorer/
-
- Siegel RL, Miller KD, Fuchs HE, et al. : Cancer statistics, 2021. CA Cancer J Clin 71:7-33, 2021 - PubMed
-
- Scher KS, Hurria A: Under-representation of older adults in cancer registration trials: Known problem, little progress. J Clin Oncol 30:2036-2038, 2012 - PubMed
-
- Talarico L, Chen G, Pazdur R: Enrollment of elderly patients in clinical trials for cancer drug registration: A 7-year experience by the US Food and Drug Administration. J Clin Oncol 22:4626-4631, 2004 - PubMed
-
- Singh H, Kanapuru B, Smith C, et al. : FDA analysis of enrollment of older adults in clinical trials for cancer drug registration: A 10-year experience by the US Food and Drug Administration. J Clin Oncol 35, 2017. (suppl 15; abstr 10009)
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

