Design and characterization of a heterobifunctional degrader of KEAP1
- PMID: 36473314
- PMCID: PMC9720105
- DOI: 10.1016/j.redox.2022.102552
Design and characterization of a heterobifunctional degrader of KEAP1
Abstract
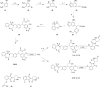
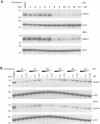
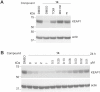
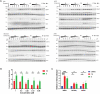
The Kelch-like ECH-associated protein 1 (KEAP1) - nuclear factor erythroid 2-related factor 2 (NRF2) signaling pathway senses reactive oxygen species and regulates cellular oxidative stress. Inhibiting KEAP1 to activate the NRF2 antioxidant response has been proposed as a promising strategy to treat chronic diseases caused by oxidative stress. Here, we developed a proteolysis targeting chimera (PROTAC) that depletes KEAP1 from cells through the ubiquitin-proteasome pathway. A previously developed KEAP1 inhibitor and thalidomide were incorporated in the heterobifunctional design of the PROTAC as ligands for KEAP1 and CRBN recruitment, respectively. Optimization of the chemical composition and linker length resulted in PROTAC 14 which exhibited potent KEAP1 degradation with low nanomolar DC50 in HEK293T (11 nM) and BEAS-2B (<1 nM) cell lines. Furthermore, PROTAC 14 increased the expression of NRF2 regulated antioxidant proteins and prevented cell death induced by reactive oxygen species. Together, these results established a blueprint for further development of KEAP1-targeted heterobifunctional degraders and will facilitate the study of the biological consequences of KEAP1 removal from cells. This approach represents an alternative therapeutic strategy to existing treatments for diseases caused by oxidative stress.
Keywords: Antioxidant; KEAP1-NRF2 pathway; Oxidative stress; PROTAC; ROS.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest All authors have approved the final version of the manuscript and declare no conflict of interest related to this work.
Figures




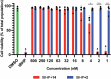
References
-
- He L., He T., Farrar S., Ji L., Liu T., Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 2017;44(2):532–553. - PubMed
-
- Wakabayashi N., Dinkova-Kostova A.T., Holtzclaw W.D., Kang M.I., Kobayashi A., Yamamoto M., Kensler T.W., Talalay P. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc. Natl. Acad. Sci. U. S. A. 2004;101(7):2040–2045. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

