Performance evaluation of the Viasure PCR assay for the diagnosis of monkeypox: A multicentre study
- PMID: 36473345
- PMCID: PMC9711911
- DOI: 10.1016/j.jcv.2022.105350
Performance evaluation of the Viasure PCR assay for the diagnosis of monkeypox: A multicentre study
Abstract
Background: Monkeypox virus (MPXV) is the causative agent of the 2022 monkeypox global outbreak. Rapid detection of MPXV infection is essential to inform patient management and public health response. Currently, there is a lack of established real-time PCR assays to support a rapid diagnosis of monkeypox.
Objectives: To evaluate the performance characteristics of the Viasure MPXV PCR assay in three London teaching hospitals.
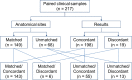
Study design: Prospectively collected paired patient swabs from matched or unmatched anatomical sites were evaluated by the reference laboratory and Viasure MPXV PCR assays. A subset of samples were also tested for HSV, VZV, and/or Treponema pallidum DNA.
Results: 217 paired samples were evaluated. 91.2% of the paired swabs generated concordant results whilst 8.8% generated discordant results. The accuracy, diagnostic sensitivity, diagnostic specificity, positive predictive value, negative predictive value, likelihood ratio positive, and likelihood ratio negative of the Viasure PCR assay across the hospitals were 93.2 - 96.3%, 90.0 - 100%, 88.2 - 100%, 94.9 - 100%, 87.9 - 100%, 8.50 - 14.41, and 0.00 - 0.10 respectively. MPXV co-infections with HSV were detected in two patients. Five patients were negative for monkeypox but positive for herpes or chickenpox.
Conclusions: The Viasure MPXV PCR assay demonstrated excellent performance characteristics, was easy to use, and is fit for routine diagnostic purpose. Where implemented, the assay would allow rapid and accurate laboratory diagnosis of MPXV infections and support a timely management of monkeypox. To reduce the risk of false negative detections, vesicular lesions from any anatomical site should be preferentially and optimally sampled.
Keywords: Diagnosis; Likelihood ratio; Monkeypox; Predictive value; Sensitivity; Specificity.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: JFT received conference registration expense from Pro-Lab Diagnostics to attend the 2022 Federation of Infection Societies Conference. All other authors have no relevant financial or non-financial competing interests to declare.
Figures
References
-
- Thornhill J.P., et al. Monkeypox virus infection in humans across 16 countries - April-June 2022. N. Engl. J. Med. 2022;387(8):679–691. - PubMed
-
- CerTest Biotec., Viasure Monkeypox virus real-time PCR detection assay (Instructions for use). 2022.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical