Lipolysis-derived linoleic acid drives beige fat progenitor cell proliferation
- PMID: 36473459
- PMCID: PMC9875052
- DOI: 10.1016/j.devcel.2022.11.007
Lipolysis-derived linoleic acid drives beige fat progenitor cell proliferation
Abstract
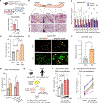
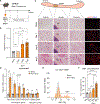
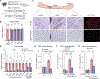
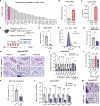
De novo beige adipocyte biogenesis involves the proliferation of progenitor cells in white adipose tissue (WAT); however, what regulates this process remains unclear. Here, we report that in mouse models but also in human tissues, WAT lipolysis-derived linoleic acid triggers beige progenitor cell proliferation following cold acclimation, β3-adrenoceptor activation, and burn injury. A subset of adipocyte progenitors, as marked by cell surface markers PDGFRα or Sca1 and CD81, harbored cristae-rich mitochondria and actively imported linoleic acid via a fatty acid transporter CD36. Linoleic acid not only was oxidized as fuel in the mitochondria but also was utilized for the synthesis of arachidonic acid-derived signaling entities such as prostaglandin D2. Oral supplementation of linoleic acid was sufficient to stimulate beige progenitor cell proliferation, even under thermoneutral conditions, in a CD36-dependent manner. Together, this study provides mechanistic insights into how diverse pathophysiological stimuli, such as cold and burn injury, promote de novo beige fat biogenesis.
Keywords: adipose tissue development; beige adipocytes; bioenergetics; brown adipose tissue; lipolysis; metabolic disease; progenitor cells; white adipose tissue.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

