Inflammation promotes synucleinopathy propagation
- PMID: 36473937
- PMCID: PMC9794777
- DOI: 10.1038/s12276-022-00895-w
Inflammation promotes synucleinopathy propagation
Erratum in
-
Correction: Inflammation promotes synucleinopathy propagation.Exp Mol Med. 2024 Apr;56(4):1029-1030. doi: 10.1038/s12276-024-01217-y. Exp Mol Med. 2024. PMID: 38556555 Free PMC article. No abstract available.
Abstract
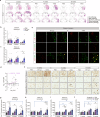
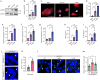
The clinical progression of neurodegenerative diseases correlates with the spread of proteinopathy in the brain. The current understanding of the mechanism of proteinopathy spread is far from complete. Here, we propose that inflammation is fundamental to proteinopathy spread. A sequence variant of α-synuclein (V40G) was much less capable of fibril formation than wild-type α-synuclein (WT-syn) and, when mixed with WT-syn, interfered with its fibrillation. However, when V40G was injected intracerebrally into mice, it induced aggregate spreading even more effectively than WT-syn. Aggregate spreading was preceded by sustained microgliosis and inflammatory responses, which were more robust with V40G than with WT-syn. Oral administration of an anti-inflammatory agent suppressed aggregate spreading, inflammation, and behavioral deficits in mice. Furthermore, exposure of cells to inflammatory cytokines increased the cell-to-cell propagation of α-synuclein. These results suggest that the inflammatory microenvironment is the major driver of the spread of synucleinopathy in the brain.
© 2022. The Author(s).
Conflict of interest statement
S-JL is a co-founder and CEO of Neuramedy Co. Ltd., and JSL is employed by Neuramedy Co. Ltd. The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

