Geometric tumor embolic budding characterizes inflammatory breast cancer
- PMID: 36473978
- PMCID: PMC9734724
- DOI: 10.1007/s10549-022-06819-6
Geometric tumor embolic budding characterizes inflammatory breast cancer
Abstract
Purpose: Inflammatory breast cancer (IBC) is characterized by numerous tumor emboli especially within dermal lymphatics. The explanation remains a mystery.
Methods: This study combines experimental studies with two different IBC xenografts with image algorithmic studies utilizing human tissue microarrays (TMAs) of IBC vs non-IBC cases to support a novel hypothesis to explain IBC's sina qua non signature of florid lymphovascular emboli.
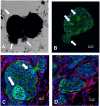
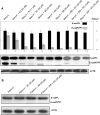
Results: In the human TMAs, compared to tumor features like nuclear grade (size), mitosis and Ki-67 immunoreactivity which show that IBC is only modestly more proliferative with larger nuclei than non-IBC, what really sets IBC apart is the markedly greater number of tumor emboli and distinctly smaller emboli whose numbers indicate geometric or exponential differences between IBC and non-IBC. In the experimental xenograft studies, Mary-X gives rise to tight spheroids in vitro which exhibit dynamic budding into smaller daughter spheroids whereas Karen-X exhibits only loose non-budding aggregates. Furthermore Mary-X emboli also bud dramatically into smaller daughter emboli in vivo. The mechanism that regulates this involves the generation of E-cad/NTF1, a calpain-mediated cleavage 100 kDa product of 120 kDa full length membrane E-cadherin. Inhibiting this calpain-mediated cleavage of E-cadherin by blocking either the calpain site of cleavage (SC) or the site of binding (SB) with specific decapeptides that both penetrate the cell membrane and mimic either the cleavage site or the binding site on E-cadherin, inhibits the generation of E-cad/NTF1 in a dose-dependent manner, reduces spheroid compactness and decreases budding.
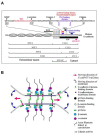
Conclusion: Since E-cad/NFT1 retains the p120ctn binding site but loses the α-and β-catenin sites, promoting its 360° distribution around the cell's membrane, the vacilating levels of this molecule trigger budding of both the spheroids as well as the emboli. Recurrent and geometric budding of parental emboli into daughter emboli then would account for the plethora of emboli seen in IBC.
Keywords: Calpain-mediated proteolysis of E-cadherin; E-cadherin fragments; IBC; Lymphovascular tumor emboli; Spheroidgenesis; Tumor embolic budding.
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors declare that they, at the present time, have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. None of the sources of support listed influenced the collection, analysis and interpretation of data, the generation of the hypothesis, the writing of the manuscript or the decision to submit the manuscript for publication.
Figures






References
-
- Kertmen N, Babacan T, Keskin O, Solak M, Sarici F, Akin S, Arik Z, Asian A, Ates O, Aksoy S, Ozisik Y, Altundag K. Molecular subtypes in patients with inflammatory breast cancer. A single center experience J BUON. 2015;20:35–39. - PubMed
-
- Alpaugh ML, Tomlinson JS, Shao ZM, Barsky SH. A novel human xenograft model of inflammatory breast cancer. Cancer Res. 1999;59:5079–5084. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

